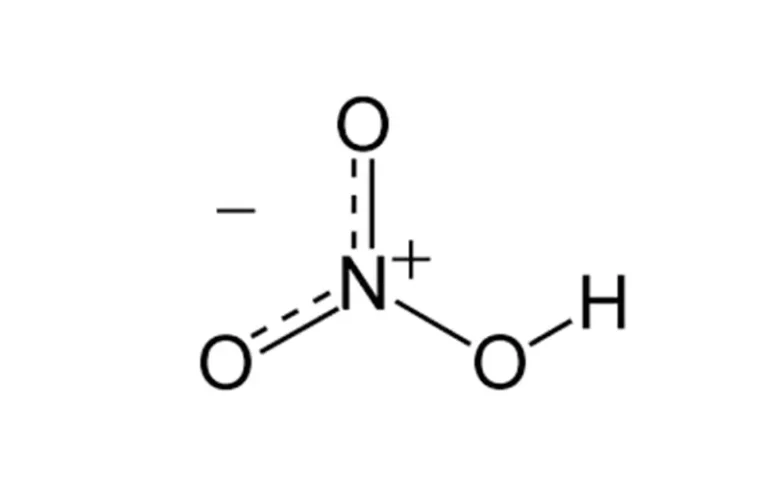
Does Ethylene Glycol Exhibit Ion-Dipole Forces?
Understanding Its Intermolecular Interactions Understanding the intermolecular forces in substances like ethylene glycol is key to many practical applications. Ethylene glycol, a polar compound, interacts through hydrogen bonding, dipole-dipole forces, and dispersion forces. Here is a table summarizing the basic information about Ethylene Glycol: Property Description Molecular Formula C₂H₆O₂ Molecular Weight 62.07 g/mol Appearance Colorless,…



.jpg)


-2-768x480.webp)