Properties
- Assay: 98%
- bp: 108-114 °C/10 mmHg (lit.)
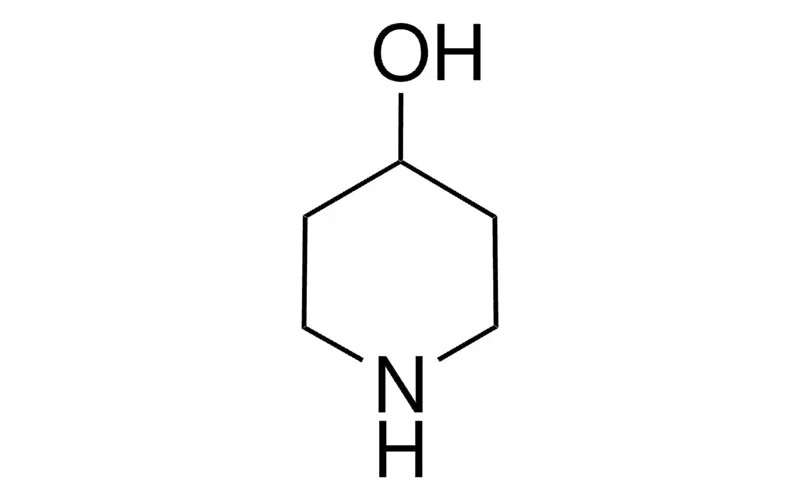
- functional group: hydroxyl
- SMILES string: OC1CCNCC1
- InChI: 1S/C5H11NO/c7-5-1-3-6-4-2-5/h5-7H,1-4H2
- InChI key: HDOWRFHMPULYOA-UHFFFAOYSA-N
Uses
4-Hydroxypiperidine is a versatile chemical used in various applications:
- Pharmaceutical Synthesis: It is used as a reagent for the synthesis of acridine derivatives and fibrinogen receptor antagonists. It is also a key intermediate in the synthesis of several pharmaceutical compounds, including potent IP (PGI(2) receptor) agonists.
- Catalysis Studies: It is used in the study of copper-catalyzed N- versus O-arylation.
- Prodrug Development: 4-Hydroxypiperidine can be used to enhance the solubility and stability of drug molecules, making it a valuable component in prodrug strategies.
Safety Data Sheet (SDS)
- Hazard Codes: Xi (Irritant), C (Corrosive).
- Risk Statements: 36/37/38 (Irritating to eyes, respiratory system, and skin); 34 (Causes burns).
- Safety Statements: 26 (In case of contact with eyes, rinse immediately with plenty of water and seek medical advice); 36/37/39 (Wear suitable protective clothing, gloves, and eye/face protection); 45 (In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible)).
- Packing Group: III.
Synthesis Methods
- Synthesis of 4-Hydroxypiperidine from 4-Piperidone:
- Step 1: 4-Piperidone hydrochloride hydrate is dissolved in distilled water, and liquid ammonia is introduced to alkalinity. The mixture is extracted with toluene, dried with anhydrous magnesium sulfate, and filtered to obtain 4-piperidone.
- Step 2: The 4-piperidone is dissolved in methanol, and sodium borohydride is added. The mixture is refluxed, concentrated, and acidified with dilute hydrochloric acid. The organic phase is separated, dried, and crystallized to obtain 4-hydroxypiperidine.
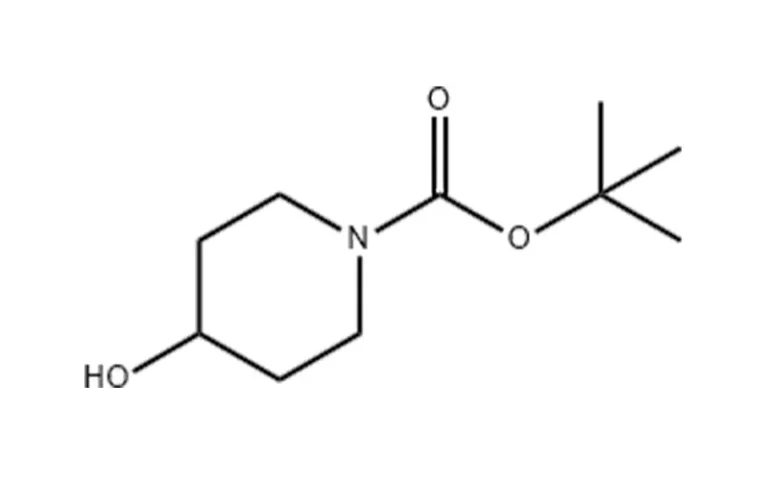
- Synthesis of N-Boc-4-Hydroxypiperidine:
- Step 1: 4-Hydroxypiperidine is dissolved in methanol, and di-tert-butyl dicarbonate is added in the presence of sodium bicarbonate. The mixture is refluxed, filtered, and crystallized to obtain N-Boc-4-hydroxypiperidine.
- Alternative Synthesis:
- A mixture of 4-hydroxypiperidine, sodium bicarbonate, and di-tert-butyl dicarbonate in dichloromethane is stirred for 15 hours. The phases are separated, and the product (N-Boc-4-hydroxypiperidine) is isolated as an oil in quantitative yield.
Handling and Storage
Use appropriate personal protective equipment (PPE) when handling this compound due to its irritant and corrosive properties.
Store in a cool, dry place, protected from moisture and light.
Our company specializes in hazardous chemicals, flammable and explosive chemicals, toxic chemicals (legal export), ultra-pure and high-purity reagents. Welcome to contact us.
Packing and shipping