Sodium Borohydride (NaBH₄)16940-66-2

I. Basic Information
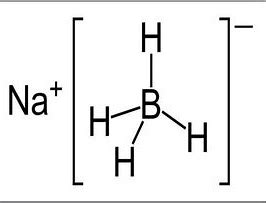
Sodium borohydride, with the chemical formula NaBH4, is a white to grayish-white crystalline powder with strong hygroscopicity.
| Item | Details |
|---|---|
| Chinese Name | Sodium Borohydride |
| English Name | Sodium borohydride |
| Alias | Sodium tetrahydroborate |
| Chemical Formula | NaBH4 |
| Molecular Weight | 37.83 |
| CAS Registry Number | 16940-66-2 |
| EINECS Registry Number | 241-004-4 |
| Melting Point | 400°C |
| Boiling Point | 500°C (decomposition) |
| Water Solubility | 550 g/L (25 ºC) |
| Density | 1.07 g/cm³ |
| Appearance | White to grayish-white crystalline powder |
| Flash Point | 70°C |
| Application | Reducing agent, foaming agent, bleaching agent |
| Safety Description | S22; S26; S36/37/39; S45 |
| Hazard Description | R15; R22; R23/24/25; R34; R43; R53 |
| UN Dangerous Goods Number | 1426 |
II. Research History
Sodium borohydride was discovered by H. C. Brown and his supervisor Schlesinger at the University of Chicago in 1942. Initially, it was to study the properties of borane and carbon monoxide complexes, but it was accidentally found that borane has the ability to reduce organic carbonyl compounds. At that time, borane was a rare substance, so it did not attract the attention of organic chemists. The development of borane chemistry benefited from World War II. The US Department of Defense needed to find a volatile uranium compound with a relatively small molecular weight for the enrichment of uranium-235. Uranium borohydride U(BH4)4 met this requirement. The synthesis of this compound requires lithium hydride.
III. Crystal Structure
Sodium borohydride has three crystal forms: α, β, and γ. At room temperature, it is a cubic crystal with the NaCl structure of the α form; it transforms into a β-type tetragonal crystal under 6.3GPa and into a γ-type orthorhombic crystal under 8.9GPa.

IV. Physical and Chemical Properties
(I) Physical Properties
Sodium borohydride is a white crystalline powder with strong hygroscopicity and is prone to deliquescence. It is soluble in water, liquid ammonia, and amines, easily soluble in methanol, slightly soluble in ethanol and tetrahydrofuran, and insoluble in ether, benzene, and hydrocarbons. It is stable in dry air, decomposes in moist air, and also decomposes when heated to 500°C.
(II) Chemical Properties
- The hydrogen in sodium borohydride has a partial negative charge (the electronegativity of B is smaller than that of H), and the hydrogen in substances such as alcohols and amines with -OH, -NH-, and -NH2 has a relatively large partial positive charge. Therefore, BH4- in sodium borohydride can form double hydrogen bonds with the molecules of these substances. As a result, sodium borohydride is soluble in water, liquid ammonia, alcohols, and amines.
- Sodium borohydride will react slowly with water and substances containing hydroxyl groups such as alcohols to release hydrogen gas. Due to the slow reaction, the loss of sodium borohydride in a short time is small. Therefore, sodium borohydride can use alkaline solutions, methanol, and ethanol as solvents. The relevant reaction equations are: NaBH4 + 2H2O = NaBO2 + 4H2↑; NaBH4 + 4HOR → B(OR)3 + 4H2↑ + NaOR.
- Sodium borohydride is an inorganic compound with strong selective reducibility and is commonly used as a reducing agent in inorganic and organic synthesis, having good chemical selectivity. It can reduce the carbonyl groups of aldehydes and ketones under mild conditions to form primary and secondary alcohols. A small amount of sodium borohydride can reduce nitriles to aldehydes, and an excess can reduce them to amines. It can selectively reduce carbonyl and aldehyde groups to hydroxyl groups, and also reduce carboxyl groups to aldehyde groups. Generally, it does not react with esters, amides, carbon-carbon double bonds, or triple bonds.
- Sodium borohydride has a good reducing effect on aldehydes and ketones. Commonly used solvents include alcohols, tetrahydrofuran, dimethylformamide (DMF), water, etc. Generally, it does not reduce ester groups and amides, but under high concentration, high temperature, with a suitable solvent or catalyzed by a Lewis acid, it can reduce weaker carbonyl groups such as ester groups.
- Sodium borohydride will decompose rapidly to produce hydrogen gas under acidic conditions and cannot exist stably. However, it is stable under neutral or alkaline conditions, and it is most stable when pH = 14. The reaction equation is: NaBH4 + H+ + 3H2O = H3BO3 + Na+ + 4H2↑.
V. Preparation Method
Sodium borohydride can be obtained by reducing trimethyl borate with sodium hydride or by reducing borax with sodium metal and hydrogen.
VI. Computational Chemical Data
| Item | Value |
|---|---|
| Reference Value of Hydrophobic Parameter Calculation (XlogP) | 0 |
| Number of Hydrogen Bond Donors | 0 |
| Number of Hydrogen Bond Acceptors | 1 |
| Number of Rotatable Chemical Bonds | 0 |
| Number of Tautomers | 0 |
| Topological Molecular Polar Surface Area | 0 |
| Number of Heavy Atoms | 2 |
| Surface Charge | 0 |
| Complexity | 2 |
| Number of Isotope Atoms | 0 |
| Number of Determined Atomic Stereocenters | 0 |
| Number of Undetermined Atomic Stereocenters | 0 |
| Number of Determined Bond Stereocenters | 0 |
| Number of Undetermined Bond Stereocenters | 0 |
| Number of Covalent Bond Units | 2 |
VII. Application Areas
Sodium borohydride used as a reducing agent for aldehydes, ketones, and acyl chlorides, as an intermediate for the production of potassium borohydride, and as a raw material for the production of diborane other high-energy fuels. In the plastic industry, it is used as a foaming agent; in the paper industry, it is used as a treatment agent for mercury-containing sewage and a paper bl eaching agent; in the medical industry, it is used as a hydrogenating agent for the production f dihydrostreptomycin. hydrogen in sodium borohydride has a valence of -1 and has strong reducibility, which can reduce inorganic substances with certain oxidability, and it is known as a “universal reducing agent”. It has stable performance and selectivity during reduction, providing organic chemists with a convenient and mild method for reducing aldehydes and ketones. However, sodium borohydride is prone to decomposition and requires maintaining the pH of the plating solution above 12. Moreover, the accumulation of its oxidation product, sodium metaborate, will have an adverse effect on the plating solution.
VIII. Related Hazards
(I) Toxicological Data
Acute toxicity: LD50 for rats by oral route: 18 mg/kg (intracoelomic in rats). The main irritating effects include corrosive effects on the skin and mucous membranes, and strong corrosive effects on the eyes. There is no known sensitization effect. The dangerous characteristics are that it can cause combustion when it comes into contact with water, moist air, acids, oxidants, high heat, and open flames. The combustion (decomposition) products are boron oxide and hydrogen.
(II) Health Hazards
Routes of entry include inhalation, ingestion, and percutaneous absorption. This product strongly irritates the mucous membranes, upper respiratory tract, eyes, and skin. Inhalation can lead to eath due to spasm, inflammation, and edema of the larynx and bronchus, chemical pneumonia, and pulmonary edema. Oral intake can corrode the digestive tract. After contact with sodium borohydride, symptoms such as sore throat, cough, rapid breathing, headache, abdominal pain, diarrhea, dizziness, conjunctival congestion, and pain may occur. Dust flying should be prevented, ventilation should be strengthened or a protective mask should be worn, and eye protection should be paid attention to. Wear closed protective glasses. It is not allowed to eat, drink water, or smoke during work. In case of poisoning, leave the site quickly, rest in a semi-recumbent position, inhale fresh air, rinse the eyes with a large amount of water, remove contaminated clothes, and rinse the whole body. For those who have inested it, rinse the mouth immediately, drink a large amount of water to induce vomiting, and then send them to the hospital for treatment. When there is a leak, carefully clean up the leaked substance while wearing a filtered protective mask.







