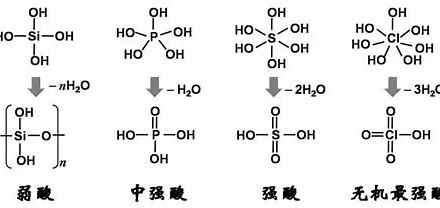
The difference between potassium dihydrogen phosphate/dipotassium hydrogen phosphate and phosphate
Do you often hear terms like Monopotassium Phosphate (MKP), Dipotassium Phosphate (DKP), or other potassium phosphate salts, and find yourself confused? These aren’t just different names; they vary significantly in composition, uses, and effects. Don’t worry!
I. Comparing the Phosphate Family Members: Monopotassium Phosphate, Tripotassium Phosphate, and Potassium Dihydrogen Phosphate Salt
The phosphate family is vast, but the most common sources of confusion typically revolve around the following. Understanding their differences will make your selection and use much more effective.
1. Monopotassium Phosphate (MKP or KH2PO4): The Versatile Phosphorus-Potassium Star
Monopotassium Phosphate is a highly favored phosphorus and potassium compound in both agriculture and industry.
- Core Composition: Contains two essential plant nutrients: phosphorus (in the dihydrogen phosphate form) and potassium.
- Key Characteristics:
- High Purity: Typically boasts high purity, is readily soluble in water, and offers excellent absorption and utilization rates.
- Slightly Acidic: Its solution is slightly acidic, making it suitable for a wide range of soil types and crops without causing excessive acidification or alkalization.
- Nitrogen-Free: Contains no nitrogen, making it ideal for phosphorus and potassium supplementation during later growth stages or specific periods, avoiding excessive vegetative growth.
- Main Uses: High-efficiency phosphorus-potassium fertilizer in agriculture, food additive (e.g., buffering agent, yeast nutrient), industrial buffering agent, etc.
- Application Comparison: In agriculture, it’s frequently used for foliar spraying, drip irrigation, or fertigation, showing remarkable effects during flower bud differentiation and fruit expansion stages.
| Category | Potassium Dihydrogen Phosphate (KH₂PO₄) | Monopotassium Phosphate (KH₂PO₄) | Potassium Dihydrogen Phosphate Salts |
|---|---|---|---|
| Properties | – White crystalline powder – Acidic salt – Highly soluble in water – pH ≈ 4.5 (1% aqueous solution) | Identical to Potassium Dihydrogen Phosphate No substantial differences | – Mixtures or double salts – Properties depend on specific components (e.g., cation types) |
| Molecular Formula | KH₂PO₄ | KH₂PO₄ | No fixed formula Examples: – Double salt: NaKH₂PO₄ – Mixture: KH₂PO₄ + other salts |
| Difference in Formula | Single pure substance containing K⁺ and H₂PO₄⁻ ions | Identical to Potassium Dihydrogen Phosphate | Contains additional cations (e.g., Na⁺, NH₄⁺) or mixed salts |
| Conversion Relationship | No relevant conversion (single substance) | No relevant conversion (same as KH₂PO₄) | Can form double salts via metathesis reactions: KH₂PO₄ + M⁺X⁻ → MKH₂PO₄ + KX (M⁺ = other cations, e.g., Na⁺, NH₄⁺) |
| Synthesis Methods | 1. Neutralization Method: H₃PO₄ + KOH → KH₂PO₄ + H₂O 2. Metathesis Method: KCl + NaH₂PO₄ → KH₂PO₄ + NaCl | Identical to Potassium Dihydrogen Phosphate | 1. Double Salt Synthesis: Mix KH₂PO₄ with solutions of other salts in proportion 2. Co-Crystallization Method: Crystallize double salts from mixed solutions |
| Main Applications | – Agriculture: High-purity phosphorus-potassium compound fertilizer (foliar fertilizer, soilless culture) – Industry: Buffering agent, brewing additive – Food: Leavening agent, flavoring agent | Identical to Potassium Dihydrogen Phosphate | – Specific fields: ・Industrial buffering systems (e.g., Na⁺-containing double salts) ・Customized fertilizers (e.g., NH₄⁺-containing N-P-K blends) ・Pharmaceutical intermediates (requires specific cations) |
| Precautions | – Avoid mixing with alkaline substances – Store in a dry environment | No additional precautions | – Clarify components (e.g., heavy metal content) – Double salts may affect solubility or pH |
| Common Aliases | Monopotassium Phosphate (common name in some industries) | Potassium Dihydrogen Phosphate (standard name) | No fixed aliases; specific naming required (e.g., sodium potassium dihydrogen phosphate, ammonium potassium dihydrogen phosphate) |
Supplementary Notes:
- Relationship between Potassium Dihydrogen Phosphate and Monopotassium Phosphate:
They are different names for the same substance. “Monopotassium Phosphate” originated from the early description of “one potassium ion per molecule of phosphoric acid,” but the standard name is now “Potassium Dihydrogen Phosphate” (referring to the combination of dihydrogen phosphate and potassium ions). - Diversity of Potassium Dihydrogen Phosphate Salts:
- Double salts (e.g., NaKH₂PO₄) contain two types of cations and can regulate solution ion strength or pH buffering range.
- Mixtures (e.g., KH₂PO₄ + KNO₃) are commonly used in compound fertilizer production for nutrient blending via physical mixing.
- Synthesis Reaction Example:
- For potassium dihydrogen phosphate: High-purity products are obtained via neutralization of phosphoric acid with potassium hydroxide, precisely controlling the pH.
- For double salts: Adding sodium carbonate to a KH₂PO₄ solution can generate sodium potassium dihydrogen phosphate (NaKH₂PO₄) and carbon dioxide:
2KH₂PO₄ + Na₂CO₃ → 2NaKH₂PO₄ + CO₂↑ + H₂O
2. Tripotassium Phosphate (TKP, K3PO4⋅H2O): Another Option for Phosphorus-Potassium Fertilization


Tripotassium Phosphate (often referred to as Potassium Phosphate Tribasic) differs significantly from Monopotassium Phosphate in both structure and properties.
- Core Composition: Contains three potassium ions and one phosphate radical.
- Key Characteristics:
- Strongly Alkaline: Its solution is strongly alkaline, with a high pH.
- Solubility: Its solubility is relatively lower compared to Monopotassium Phosphate.
- Main Uses: Primarily used as a fertilizer, but also finds applications in specific industrial fields.
- Application Comparison: Due to its strong alkalinity, its agricultural use requires careful consideration of soil pH, making it more suitable for acidic soils to avoid further increasing soil alkalinity.
3. Potassium Dihydrogen Phosphate Salt (Typically Refers to MKP, KH2PO4): Clarifying Names and Confusions
The term “Potassium Dihydrogen Phosphate Salt” usually refers to Monopotassium Phosphate (MKP or KH2PO4). It’s not a distinct compound entirely different from MKP, but rather an alternative name used in certain contexts.
- Common Market Misconception: It’s sometimes confused with “Dipotassium Phosphate (DKP or K2HPO4).”
- Dipotassium Phosphate (DKP or K2HPO4) Brief Analysis:
- Core Composition: Contains two potassium ions and one hydrogen phosphate radical.
- Key Characteristics: Its solution is mildly alkaline, often serving as a buffering agent or pharmaceutical intermediate.
- Agricultural Application: Compared to MKP, its widespread use as a fertilizer in agriculture is less common.
- Dipotassium Phosphate (DKP or K2HPO4) Brief Analysis:
- Summary Comparison:
- Acidity/Alkalinity: Monopotassium Phosphate (slightly acidic) < Dipotassium Phosphate (mildly alkaline) < Tripotassium Phosphate (strongly alkaline).
- Agricultural Application: Monopotassium Phosphate is the preferred choice for agricultural phosphorus-potassium fertilization due to its high purity, easy absorption, and slightly acidic nature.
- Professional Selection: When purchasing or using, always select the correct potassium phosphate product based on your specific needs (soil type, crop growth stage, industry requirements) and product specifications.

II. Unveiling Monopotassium Phosphate’s “Golden Ratios”: Application Examples Across Various Industries
Monopotassium Phosphate plays a crucial role in multiple industries due to its excellent properties, and precise application ratios are key to maximizing its effectiveness.
1. Agriculture: The Secret Weapon for High Yields
Monopotassium Phosphate is widely recognized as a “yield-boosting miracle” in agriculture, primarily applied through foliar spraying, drip irrigation, or fertigation.
- Foliar Spraying:
- Common Concentration: Typically 0.2% – 0.3% (i.e., add 0.2-0.3 kg of MKP per 100 kg of water).
- Application Examples:
- Fruit Trees: Spraying before flowering and during fruit expansion can significantly promote flower bud differentiation, increase fruit set, and improve fruit quality and coloring.
- Vegetables: Spraying during flowering and fruiting can effectively boost yields and enhance disease resistance.
- Field Crops (e.g., wheat, corn): Spraying during the grain-filling stage can substantially increase grain weight and improve thousand-grain weight.
- Drip Irrigation/Fertigation:
- Common Concentration: 0.05% – 0.1% (adjusted based on crop type, growth stage, and soil fertility).
- Application Examples: Widely used in protected agriculture or for high-value crops to provide continuous phosphorus and potassium nutrition.
- Seed Treatment:
- Common Concentration: Soaking seeds in a 0.1% – 0.5% solution.
- Application Examples: Can significantly improve seed germination rates and seedling stress resistance, laying a solid foundation for crop growth.
2. Food Industry: A Safe and Efficient “Magical” Additive
Monopotassium Phosphate plays a vital role in food processing, often serving as a safe food additive.
- As a Buffering Agent/Acidity Regulator:
- Addition Ratio: Typically between 0.1% – 0.5% (depending on the food type and regulatory requirements).
- Application Examples:
- Beverages: Used to adjust pH, effectively improving taste and extending product shelf life.
- Baked Goods: As a buffering agent for leavening agents, it stabilizes dough pH, thereby influencing the final product’s taste and texture.
- Dairy Products: Prevents protein coagulation, maintaining product stability.
- As a Yeast Nutrient:
- Addition Ratio: Usually between 0.01% – 0.05%.
- Application Examples: Effectively promotes yeast proliferation and fermentation activity, widely used in brewing, bread making, and other processes.
3. Pharmaceutical Industry: A Key Component in Pharmaceuticals and Healthcare
In the pharmaceutical field, the purity and stability of Monopotassium Phosphate are paramount.
- As a pH Adjuster/Buffering Agent:
- Addition Ratio: Precisely adjusted based on the drug’s dosage form and desired pH range, typically in trace amounts.
- Application Examples:
- Injections: Maintains the pH stability of drug solutions, ensuring drug stability and efficacy.
- Oral Solutions/Eye Drops: Adjusted to a physiological pH to reduce irritation to the patient.
- As a Phosphorus Supplement:
- Addition Ratio: In clinical applications, Monopotassium Phosphate is often used to formulate intravenous infusions to supplement phosphorus in patients. The dosage must be strictly followed as prescribed by a doctor; self-administration is not permitted.
- Application Examples: Used in the treatment of hypophosphatemia, among other conditions.
4. Other Industrial Applications: From Fire Retardants to Electroplating
The uses of Monopotassium Phosphate extend beyond these, with broad applications in other industrial fields.
- Fire Retardant: As a component of intumescent fire retardant coatings, its proportion is determined by the specific product formulation, effectively enhancing the material’s flame resistance.
- Electroplating Solution: Functions as a buffering and complexing agent in electroplating solutions, helping to stabilize solution performance and improve the uniformity and quality of the plating layer.
III. Price Perspective: Analysis of Monopotassium Phosphate Price Increases in Recent Years
The market price of Monopotassium Phosphate is not static; it is influenced by a complex interplay of various factors.
- Key Factors Affecting Price:
- Raw Material Costs: Fluctuations in the prices of upstream raw materials like phosphate rock and potash salts directly impact the production cost of MKP.
- Supply and Demand: Increased demand during peak agricultural seasons may drive up prices; conversely, market oversupply can lead to price declines.
- Energy Costs: Changes in the prices of electricity, natural gas, and other energy sources required during production are also significant cost factors.
- International Market Environment: Macro factors such as international trade policies, exchange rate fluctuations, and geopolitical events can all influence the global price of MKP.
- Environmental Policies: Increasingly stringent environmental regulations may limit certain production capacities, affecting market supply and prices.
- Recent Price Trends (Based on general market conditions; specific data requires real-time quotes):
- In recent years, the price of Monopotassium Phosphate has shown a degree of volatility. For example, in 2022 and 2023, influenced by rising global agricultural product prices and surging international energy costs, MKP prices experienced significant increases, with some periods seeing rises of 20%-40%. However, since 2024, with the gradual stabilization of supply chains and adjustments in market supply and demand, prices may have retreated slightly or stabilized.
- (Please note: This is a general trend description; specific years and percentage increases require up-to-date market data.)
- Purchasing Advice: Given market volatility, it is advisable for consumers and businesses to monitor real-time market prices, choose reputable and stable suppliers, and plan their procurement volumes in advance to secure more favorable prices.