Benzoyl Peroxide/94-36-0/C₁₄H₁₀O₄
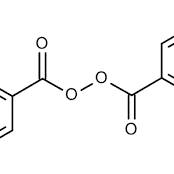
Dibenzoyl peroxide, also known as benzoyl peroxide and commonly named initiator BPO, is an organic compound. Its chemical formula is C₁₄H₁₀O₄. At room temperature, it is a white crystalline powder with a slight bitter almond odor. It is soluble in benzene, chloroform, and ether, and slightly soluble in ethanol and water. It is mainly used as a monomer polymerization initiator for polyvinyl chloride, unsaturated polyesters, polyacrylates, etc., can also be used as a cross-linking agent for polyethylene, and can also be used as a rubber vulcanizing agent. On October 27, 2017, the list of carcinogens preliminarily compiled by the International Agency for Research on Cancer of the World Health Organization indicates that dibenzoyl peroxide is in the list of category 3 carcinogens.
Basic Information
| Item | Details |
|---|---|
| Chinese Name | Dibenzoyl peroxide |
| English Name | Dibenzoyl peroxide; Benzoyl Peroxide |
| Alias | Benzoyl peroxide, Initiator BPO |
| Chemical Formula | C₁₄H₁₀O₄ |
| Molecular Weight | 242.23 |
| CAS Registry Number | 94-36-0 |
| EINECS Number | 202-327-6 |
| Melting Point | 105℃ |
| Boiling Point | 349.7℃ |
| Water Solubility | Slightly soluble |
| Density | 1.334 g/cm³ |
| Appearance | White crystalline powder |
| Flash Point | 154.2℃ |
| UN Dangerous Goods Number | 3108 |
Physical and Chemical Properties
Chemical Properties
Dibenzoyl peroxide is a strong oxidant and is flammable. Its properties are extremely unstable. Friction, impact, exposure to bright light, high temperature, sulfur, and reducing agents, etc., all pose a risk of ignition and explosion. When sulfuric acid is added, combustion can also be triggered. To prevent explosion, it is generally diluted to about 20% with insoluble salts such as calcium carbonate, calcium phosphate, calcium sulfate, or substances like talcum powder and bentonite. Alternatively, 25-30% water should be added during storage.
Molecular Structure Data
| Item | Details |
|---|---|
| Molar Refraction | 64.73 |
| Molar Volume (cm³/mol) | 193.1 |
| Parachor (90.2K) | 512.5 |
| Surface Tension (dyne/cm) | 49.6 |
| Polarizability (10⁻²⁴cm³) | 25.66 |
Computational Chemical Data
| Item | Details |
|---|---|
| Calculated Reference Value of Hydrophobic Parameter (XlogP) | 3.5 |
| Number of Hydrogen Bond Donors | 0 |
| Number of Hydrogen Bond Acceptors | 4 |
| Number of Rotatable Chemical Bonds | 5 |
| Number of Tautomers | 0 |
| Topological Molecular Polar Surface Area | 52.6 |
| Number of Heavy Atoms | 18 |
| Surface Charge | 0 |
| Complexity | 258 |
| Number of Isotope Atoms | 0 |
| Number of Determined Atomic Stereocenters | 0 |
| Number of Undetermined Atomic Stereocenters | 0 |
| Number of Determined Chemical Bond Stereocenters | 0 |
| Number of Undetermined Chemical Bond Stereocenters | 0 |
| Number of Covalent Bond Units | 1 |

Toxicological Data
- Acute Toxicity: LD50: 7710mg/kg (orally administered to rats)
- Irritation: Applied to rabbits’ eyes: 500mg (for 24 hours), mild irritation.
Ecological Data
- Biodegradability:
- Aerobic biodegradation: 24~168h
- Anaerobic biodegradation: 96~672h
- Abiotic Degradation:
- Maximum absorption wavelength range of photolysis: 235~275nm
- Atmospheric photodegradation half-life: 51~510h
- Bioconcentration Factor: BCF: 250 (theoretical)
Preparation Method
Under cooling conditions, 30% hydrogen peroxide is added to a 30% sodium hydroxide solution to form a sodium peroxide solution; then benzoyl chloride is added dropwise with stirring at 0~10℃. Excessive temperature will cause the decomposition of hydrogen peroxide and the hydrolysis of benzoyl chloride; the dibenzoyl peroxide formed in the reaction is precipitated, cooled, filtered, washed, recrystallized with a 2:1 methanol/chloroform mixture, and dried (at 50~70℃) to obtain the product with a yield of over 85%.
Relevant reaction equations:
2NaOH + H₂O₂ → Na₂O₂ + 2H₂O
Na₂O₂ + 2C₆H₅COCl → C₆H₅COOOOC₆H₅ + 2NaCl
Another method: React hydrogen peroxide with a 30% alkali solution to form a sodium peroxide solution, and then react with benzoyl chloride to obtain the product. The reaction is carried out at around 0℃. Excessive temperature will cause the decomposition of hydrogen peroxide, and benzoyl chloride is also prone to hydrolysis to form benzoic acid, affecting the yield. The precipitated dibenzoyl peroxide is filtered, washed, and dried to obtain the finished product. The content of industrial-grade dibenzoyl peroxide can reach 99% (second-grade product), with a melting point of 102-106℃. The raw material consumption quota: benzoyl chloride (above 95%) 1000kg/t, hydrogen peroxide (30%) 800kg/t. When purification is required, it can be recrystallized with alcohols, acetone, benzene, and other suitable solvents.
Pharmacopoeia Information
Basic Information
This product is hydrated dibenzoyl peroxide, containing C₁₄H₁₀O₄ should be 70.0%~77.0%, and the water content should not be less than 20.0%.
Properties
This product is a white crystalline powder with a special odor.
This product is easily soluble in acetone, slightly soluble in methanol or ethanol, and extremely slightly soluble in water.
Identification
- Take this product, dissolve it in absolute ethanol and dilute it to prepare a solution containing approximately 5µg per 1mL. Determine it according to the UV-Vis spectrophotometry (General Rule 0401), and there should be a maximum absorption at a wavelength of 235nm.
- The infrared absorption spectrum of this product should be consistent with the control spectrum (Spectral Collection 602).
Inspection
Chloride
Weigh 0.5g of the sample precisely. Dissolve it in 15mL of acetone. Then, while shaking, slowly add 50mL of 0.05mol/L nitric acid solution. Let it stand for 10 minutes and filter. Transfer the filtrate to a 100mL volumetric flask. Wash the filter residue twice with 10mL of 0.05mol/L nitric acid solution each time, combine the washings with the filtrate, and dilute to the mark with 0.05mol/L nitric acid solution. Shake well. Take 10mL of this solution, dilute it to 30mL with water, and inspect it according to the method specified in General Rule 0801. Compare it with the control solution prepared from 3.5mL of standard sodium chloride solution. The sample solution should not be more concentrated than the control solution (0.07%).
Related Substances
Determination is carried out according to High Performance Liquid Chromatography (General Rule 0512).
- Test Solution: Weigh an appropriate amount of the sample precisely. Dissolve it in acetonitrile and dilute quantitatively to prepare a solution containing approximately 2mg of dibenzoyl peroxide per 1mL.
- Reference Solution: Pipette an appropriate volume of the test solution accurately and dilute it quantitatively with acetonitrile to prepare a solution containing 2µg per 1mL.
- Reference Substance Solution: Weigh appropriate amounts of benzoic acid reference substance, benzaldehyde reference substance, and ethyl benzoate reference substance precisely. Dissolve them in the mobile phase and dilute quantitatively to prepare a mixed solution containing 150µg, 25µg, and 25µg per 1mL respectively. Then pipette an appropriate volume of this mixed solution and dilute it quantitatively with acetonitrile to prepare a solution containing 30µg, 5µg, and 5µg per 1mL respectively.
- Chromatographic Conditions: Use octadecylsilane bonded silica gel as the packing material. The mobile phase is a mixture of acetonitrile – water – glacial acetic acid (500:500:1). The detection wavelength is 235nm, and the injection volume is 20μL.
- System Suitability Requirements: In the chromatogram of the reference substance solution, the resolution between the benzaldehyde peak and the benzoic acid peak should be greater than 6.0.
- Determination Method: Pipette the test solution, reference solution, and reference substance solution precisely and inject them into the liquid chromatograph respectively. Record the chromatogram until 2 times the retention time of the main component peak.
- Limit: If there are chromatographic peaks in the chromatogram of the test solution with the same retention time as those of benzoic acid, benzaldehyde, and ethyl benzoate, calculate their areas by the external standard method. The contents should not exceed 1.5%, 0.25%, and 0.25% respectively. The sum of the areas of other impurity peaks should not be greater than 10 times the area of the main peak in the reference solution (1.0%). The total amount of impurities should not exceed 2.0%. Chromatographic peaks with an area less than 0.2 times that of the main peak in the reference solution are ignored.
Content Determination
Anhydrous Dibenzoyl Peroxide
Weigh 0.25g of the sample precisely and place it in a 240mL iodine flask. Add 30mL of acetone and shake to dissolve it. Then add 5mL of potassium iodide test solution. Seal the flask tightly, shake well, and place it in the dark for 15 minutes. Titrate with sodium thiosulfate titrant (0.1mol/L) until the solution becomes colorless, and correct the result by a blank test. Each 1mL of sodium thiosulfate titrant (0.1mol/L) corresponds to 12.11mg of C₁₄H₁₀O₄.
Water
Weigh 0.12g of the sample precisely. Dissolve it in 5mL of N,N – dimethylformamide. Add 20mL of anhydrous methanol and 3mL of a 10% potassium iodide solution in N,N – dimethylformamide. Stir for 5 minutes. Determine the water content according to the Karl Fischer Titration Method (General Rule 0832, Method 1). Add the product of the content of anhydrous dibenzoyl peroxide and 0.0744 to the measured result to obtain the water content of the sample.
Category
Disinfectant and Antiseptic.
Storage
Store in a cool, dry place protected from light and seal tightly. Ensure a certain amount of moisture is maintained during storage.
Preparations
- Benzoyl Peroxide Cream
- Benzoyl Peroxide Gel
Dibenzoyl peroxide, with its specific inspection methods, content determination procedures, and storage requirements, plays an important role in various applications. Understanding these details is crucial for ensuring the quality, safety, and effectiveness of products containing this substance. Whether in industrial production or pharmaceutical applications, strict compliance with these specifications helps maintain high – quality standards and reduces potential risks.







