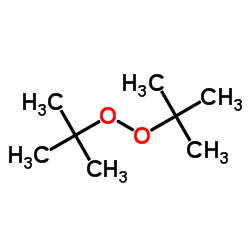
Di-tert-butyl Peroxide (DTBP)/110-05-4/C8H18O2
In the chemical industry, Di-tert-butyl peroxide (abbreviated as DTBP) is a chemical substance that has attracted much attention. Its CAS number is 110-05-4, and its molecular formula is C₈H₁₈O₂, with a molecular weight of 146.23. It also has several other aliases, such as di-tert-butyl peroxide, bis(1,1-dimethyl)peroxide, etc., and plays an important role in industrial production
| Classification | Details |
|---|---|
| CAS Number | 110-05-4 |
| MDL Number | MFCD00008803 |
| EINECS Number | 203-733-6 |
| RTECS Number | ER2450000 |
| BRN Number | 1735581 |
| PubChem Number | 24850244 |
| Physical Property Data | Appearance: Colorless liquid |
| Physical Property Data | Density (g/mL, 20°C): 0.7940 |
| Physical Property Data | Relative vapor density (g/mL, air = 1): 5.03 |
| Physical Property Data | Melting point (°C): -40 |
| Physical Property Data | Boiling point (°C, at normal pressure): 110 |
| Physical Property Data | Boiling point (°C, at 38 kPa): 80 |
| Physical Property Data | Refractive index: 1.2890 |
| Physical Property Data | Flash point (°C): 18 |
| Physical Property Data | Refractive index at room temperature (n20): 1.3872 |
| Physical Property Data | Refractive index at room temperature (n25): 1.3838 |
| Physical Property Data | Vapor pressure (mmHg, 20°C): 40 |
| Physical Property Data | Saturated vapor pressure (kPa, 20°C): 2.59 |
| Physical Property Data | Solubility parameter (J·cm⁻³)⁰.⁵: 14.182 |
| Physical Property Data | Van der Waals area (cm²·mol⁻¹): 1.392×10¹⁰ |
| Physical Property Data | Van der Waals volume (cm³·mol⁻¹): 96.080 |
| Physical Property Data | Standard enthalpy of combustion in the gas phase (kJ·mol⁻¹): -5371.4 |
| Physical Property Data | Standard enthalpy of formation in the gas phase (kJ·mol⁻¹): -349.1 |
| Physical Property Data | Standard enthalpy of combustion in the liquid phase (kJ·mol⁻¹): -5339.6 |
| Physical Property Data | Solubility: Miscible with organic solvents such as benzene and petroleum ether, insoluble in water |
| Physical Property Data | Theoretical active oxygen: 10.94% |
| Physical Property Data | Half-life: 218 h (100°C), 34 h (115°C), 0.15 h (130°C) |
| Physical Property Data | Standard enthalpy of formation in the liquid phase (kJ·mol⁻¹): -380.9 |
| Physical Property Data | Standard molar heat capacity in the liquid phase (J·mol⁻¹·K⁻¹): 296.7 |
| Toxicological Data | This product is slightly toxic and can irritate the eyes, skin and respiratory tract. |
| Ecological Data | This substance may be harmful to the environment and special attention should be paid to water bodies. |
| Molecular Structure Data | Molar refraction: 42.53 |
| Molecular Structure Data | Molar volume (cm³/mol): 173.4 |
| Molecular Structure Data | Parachor (90.2 K): 379.9 |
| Molecular Structure Data | Surface tension (dyne/cm): 23.0 |
| Molecular Structure Data | Polarizability (10⁻²⁴ cm³): 16.86 |
II. Properties and Stability of Di-tert-butyl Peroxide
Di-tert-butyl peroxide has strong oxidizing properties and is flammable. It is relatively stable at room temperature and insensitive to impact, but this does not mean that its hazard can be ignored. It is prohibited from contacting with strong reducing agents and strong alkalis. Once it comes into contact with reducing agents or is impacted, there is a risk of explosion. Therefore, during the storage and use process, relevant specifications must be strictly followed.
III. Storage Method of Di-tert-butyl Peroxide
To ensure the storage safety of di-tert-butyl peroxide, it should be stored in a cool and ventilated warehouse, away from ignition sources and heat sources, and direct sunlight should be avoided. The warehouse temperature should not exceed 30°C. At the same time, the container should be kept sealed, and it should be stored separately from reducing agents and alkalis, and mixed storage is strictly prohibited. The warehouse should be equipped with explosion-proof lighting and ventilation facilities, and mechanical equipment and tools that are prone to generating sparks are prohibited. In addition, the storage area should also be equipped with leakage emergency treatment equipment and suitable containment materials, and vibration, impact, and friction are strictly prohibited.
IV. Synthesis Method of Di-tert-butyl Peroxide
Classic Synthesis Method
Add 222g of tert-butanol and 140g of 70% sulfuric acid to an enamel reaction kettle, stir and mix, and cool to 2~ – 8°C. Under vigorous stirring, add 126g of 27% hydrogen peroxide and 400g of concentrated sulfuric acid dropwise within 90 minutes. After adding, continue stirring for 3 hours. Let it stand and separate the oil layer. Wash it with water, remove tert-butyl hydroperoxide with a 30% sodium hydroxide solution, wash it with water again, dry it with magnesium sulfate, and filter to obtain the finished product.
Laboratory Preparation Method
Add 222g (3.0mol) of tert-butanol (2) and 140g of 70% sulfuric acid to a reaction flask equipped with a stirrer, thermometer, and dropping funnel. Stir and cool to – 2~ – 8°C. Slowly add 126g (1.0mol) of 27% hydrogen peroxide and 400g (4.0mol) of concentrated sulfuric acid dropwise, which is about 1.5 hours for the addition. After the addition, continue stirring for 3 hours. Separate the oil layer, wash it with water, remove tert-butyl hydroperoxide with a 30% sodium hydroxide solution, wash it with water, dry it with anhydrous magnesium sulfate, and filter to obtain di-tert-butyl peroxide. It should be noted that its theoretical active oxygen content is 10.94%, and safety must be paid attention to during preparation and use.
V. Applications of Di-tert-butyl Peroxide
Di-tert-butyl peroxide has a wide range of applications in multiple fields. In the chemical synthesis field, it is commonly used as an initiator for synthetic resins, which can initiate polymerization reactions and promote the synthesis of resins. As a photosensitizer for photopolymerization, it can enhance the efficiency of photopolymerization reactions. In the rubber industry, it is an important rubber vulcanizing agent, which can improve the properties of rubber and the quality of rubber products. In the fuel field, it can be used as a diesel ignition promoter to improve the combustion performance of diesel. In addition, it is also used in organic synthesis, playing a key role in the cross-linking process of unsaturated polyesters and silicone rubbers. It can also be used as an additive for diesel and an anti-gelling agent for transformer oil to improve the performance of oils.

VI. Safety Information of Di-tert-butyl Peroxide
Di-tert-butyl peroxide is a hazardous chemical, and its dangerous transportation code is UN 3107 5.2. It has hazard symbols of F (flammable) and O (oxidizing agent). In terms of safe operation, safety signs S14 (keep away from incompatible substances), S16 (keep away from fire sources), S36/S37/S39 (wear appropriate protective clothing, gloves and goggles/mask) need to be followed. At the same time, it also has hazard signs R7 (may cause fire) and R11 (highly flammable). Therefore, safety must be highly emphasized during the use and transportation process, and relevant regulations must be strictly complied with to prevent accidents.
In conclusion, di-tert-butyl peroxide occupies an important position in the chemical industry due to its unique chemical properties and wide application fields. However, during the use process, its characteristics must be fully understood, and safety specifications must be strictly followed to ensure the safety of production and use.