Hydrogen Cyanide (HCN)74-90-8
| Item | Details |
|---|---|
| Chinese Name | Hydrogen Cyanide |
| English Name | hydrogen cyanide |
| Alias | Methanenitrile |
| Chemical Formula | HCN |
| Molecular Weight | 27.03 |
| CAS Registry Number | 74 – 90 – 8 |
| EINECS Registry Number | 200 – 821 – 6 |
| Melting Point | -13.4℃ |
| Boiling Point | 26℃ |
| Water Solubility | Soluble |
| Density | 0.69g/cm³ |
| Appearance | Colorless gas or liquid with a bitter almond odor |
| Flash Point | -17.8℃ |
| Application Areas | Electroplating industry, mining industry, etc. |
| Safety Descriptions | S16; S36/37; S38; S45 |
| Hazard Symbols | T+; N; F |
| Hazard Descriptions | R12; R23/24/25; R26 |
| UN Dangerous Goods Number | 1051 |
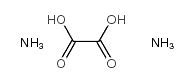
II. Detailed Explanation of Physical and Chemical Properties
(I) Physical Properties
| Property | Value |
|---|---|
| Density | 0.69g/cm³ |
| Melting Point | -13.4℃ |
| Boiling Point | 26℃ |
| Flash Point | -17.8℃ |
| Saturated Vapor Pressure | 82.46kPa (at 20℃) |
| Critical Pressure | 4.95MPa |
| Critical Temperature | 183.5℃ |
| LogP | -0.25 |
| Refractive Index | 1.531 (at 20℃) |
| Explosion Limit | 5.6% – 40% (by volume) |
| Appearance | Colorless transparent liquid, volatile, with a bitter almond odor |
| Solubility | Miscible with ethanol, ether, glycerol, ammonia, benzene, chloroform, water, etc. |
(II) Chemical Properties
Hydrogen cyanide is a weak acid that can react with bases to form salts. When its aqueous solution boils, partial hydrolysis occurs to produce ammonium formate. Under alkaline conditions, it can combine with aldehydes and ketones to form cyanohydrins, and react with acetone to produce acetone cyanohydrin. Gaseous hydrogen cyanide generally does not polymerize, but polymerization may occur when there is water condensation, and oxygen does not promote the polymerization. Liquid hydrogen cyanide or its aqueous solution will initiate polymerization under conditions such as alkalinity, high temperature, long – term storage, exposure to light and radiation, electrical discharge, and electrolysis. Once polymerization starts, the generated heat will trigger a chain reaction, accelerating the polymerization process and releasing a large amount of heat energy, which may lead to a violent explosion. The explosion limit is 5.6% – 40% (by volume). Its vapor burns with a blue flame.
When hydrogen cyanide is present in the air, it can be detected using a variety of test papers: the benzidine – copper acetate test paper shows a blue reaction; the methyl orange – mercury (II) chloride test paper changes from orange to pink; the picric acid – sodium carbonate test paper changes from yellow to brown. Hydrogen cyanide is highly toxic. Since its acidity is weaker than that of carbonic acid and stronger than that of bicarbonate, it cannot react with carbonates to release CO₂. Instead, cyanides will absorb CO₂ and form bicarbonates.
III. Molecular Structure and Computational Chemical Data
(I) Molecular Structure Data
| Item | Value |
|---|---|
| Molar Refraction | 6.41 |
| Molar Volume (cm³/mol) | 38.8 |
| Parachor (90.2K) | 38.8 |
| Surface Tension (dyne/cm) | 18.8 |
| Polarizability (10⁻²⁴cm³) | 2.54 |
(II) Computational Chemical Data
| Item | Value |
|---|---|
| Reference Value of Hydrophobic Parameter Calculation (XlogP) | 0.1 |
| Number of Hydrogen Bond Donors | 0 |
| Number of Hydrogen Bond Acceptors | 1 |
| Number of Rotatable Chemical Bonds | 0 |
| Number of Tautomers | 2 |
| Topological Molecular Polar Surface Area | 23.8 |
| Number of Heavy Atoms | 2 |
| Surface Charge | 0 |
| Complexity | 10 |
| Number of Isotope Atoms | 0 |
| Number of Determined Atomic Stereocenters | 0 |
| Number of Undetermined Atomic Stereocenters | 0 |
| Number of Determined Bond Stereocenters | 0 |
| Number of Undetermined Bond Stereocenters | 0 |
| Number of Covalent Bond Units | 1 |
IV. Toxicological Research
(I) Toxicity Tests
| Number | Toxicity Type | Testing Method | Test Object | Dosage Used | Toxic Effects |
|---|---|---|---|---|---|
| 1 | Acute | Oral | Human | 570 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 2 | Acute | Inhalation | Adult Male | 500 mg/m³/3M-C | Ocular toxicity – pupil dilation; Behavioral toxicity – coma; Lung, chest or respiratory toxicity – respiratory depression |
| 3 | Acute | Inhalation | Human | 120 mg/m³/1H | No other lethal dose values reported except for detailed toxic side effects |
| 4 | Acute | Inhalation | Human | 200 mg/m³/10M | Behavioral toxicity – general anesthesia; Lung, chest or respiratory toxicity – dyspnea; Gastrointestinal toxicity – nausea, vomiting |
| 5 | Acute | Inhalation | Adult Male | 400 mg/m³/2M | No other lethal dose values reported except for detailed toxic side effects |
| 6 | Acute | Subcutaneous Injection | Human | 1 mg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 7 | Acute | Intravenous Injection | Adult Male | 55 μg/kg | Lung, chest or respiratory toxicity – irritate the respiratory tract |
| 8 | Acute | Unreported | Adult Male | 1471 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 9 | Acute | Inhalation | Rat | 160 ppm/30M | No other lethal dose values reported except for detailed toxic side effects |
| 10 | Acute | Intravenous Injection | Rat | 810 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 11 | Acute | Oral | Mouse | 3700 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 12 | Acute | Inhalation | Mouse | 323 ppm/5M | No other lethal dose values reported except for detailed toxic side effects |
| 13 | Acute | Intraperitoneal Injection | Mouse | 2990 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 14 | Acute | Subcutaneous Injection | Mouse | 3 mg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 15 | Acute | Intravenous Injection | Mouse | 990 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 16 | Acute | Intramuscular Injection | Mouse | 2700 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 17 | Acute | Oral | Dog | 4 mg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 18 | Acute | Subcutaneous Injection | Dog | 1700 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 19 | Acute | Intravenous Injection | Dog | 1340 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 20 | Acute | Intravenous Injection | Monkey | 1300 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 21 | Acute | Subcutaneous Injection | Cat | 1100 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 22 | Acute | Intravenous Injection | Cat | 810 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 23 | Acute | Oral | Rabbit | 4 mg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 24 | Acute | Inhalation | Rabbit | 208 mg/m³/35M | Brain toxicity – other degenerative changes; Cardiac toxicity – other changes; Blood toxicity – other changes |
| 25 | Acute | Intraperitoneal Injection | Rabbit | 1570 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 26 | Acute | Subcutaneous Injection | Rabbit | 2500 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 27 | Acute | Intravenous Injection | Rabbit | 660 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 28 | Acute | Intramuscular Injection | Rabbit | 486 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 29 | Acute | Ocular Injection | Rabbit | 1040 μg/kg | Ocular toxicity – not reported; Behavioral toxicity – ataxia; Lung, chest or respiratory toxicity – irritate the respiratory tract |
| 30 | Acute | Oral | Pig | 2 mg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 31 | Acute | Subcutaneous Injection | Guinea Pig | 100 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 32 | Acute | Intravenous Injection | Guinea Pig | 1430 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 33 | Acute | Oral | Pigeon | 14 mg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 34 | Acute | Subcutaneous Injection | Pigeon | 2150 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 35 | Acute | Intramuscular Injection | Pigeon | 1500 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 36 | Acute | Oral | Duck | 3280 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 37 | Acute | Subcutaneous Injection | Frog | 60 mg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 38 | Acute | Intravenous Injection | Domestic Mammal | 660 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 39 | Acute | Inhalation | Mammal | 200 ppm/5M | No other lethal dose values reported except for detailed toxic side effects |
| 40 | Acute | Inhalation | Mammal | 36 ppm/2H | No other lethal dose values reported except for detailed toxic side effects |
| 41 | Acute | Oral | Domestic Poultry | 600 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 42 | Acute | Oral | Wild Bird | 7500 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 43 | Acute | Subcutaneous Injection | Wild Bird | 100 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
| 44 | Acute | Subcutaneous Injection | Domestic Poultry | 100 μg/kg | No other lethal dose values reported except for detailed toxic side effects |
(II) Toxicological Data
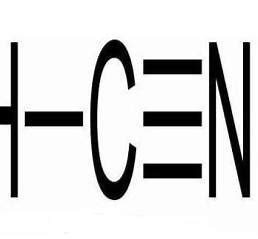
The maximum allowable concentration of hydrogen cyanide is 0.3mg/m³, and the lethal dose is 1mg/kg (body weight). The acute toxicity LC50 is 357mg/m³ (inhalation by mice for 5 minutes). Cyanide ions can inhibit the activity of 42 enzymes in tissue cells. Among them, cytochrome oxidase is the most sensitive to cyanides. Cyanide ions rapidly combine with Fe³⁺ in oxidized cytochrome oxidase, preventing its reduction to Fe²⁺ and interrupting the oxidation process of electron transfer. This leads to tissue cells being unable to utilize the oxygen in the blood, causing internal asphyxia. Since the central nervous system is the most sensitive to hypoxia, the brain is damaged first, which in turn triggers central respiratory failure and death. In addition, the hydroxide ions released by cyanides in the digestive tract have a corrosive effect. Inhalation of high – concentration hydrogen cyanide or ingestion of a large amount of cyanides can cause respiratory arrest within 2 – 3 minutes, presenting as “electroshock – like” death. Cyanide ions combine with Fe²⁺ in the blood to form [Fe(CN)₆]⁴⁻, reducing the oxygen – carrying capacity of the blood (6CN⁻ + Fe²⁺ = [Fe(CN)₆]⁴⁻).
The hazards of cyanides to the human body include acute poisoning and chronic effects. Acute poisoning is divided into three levels: mild, moderate, and severe. Mild poisoning is manifested as irritation symptoms of the eyes and upper respiratory tract, a bitter almond smell, numbness of the lips and pharynx, accompanied by nausea, vomiting, tremors, etc.; moderate poisoning is manifested as sighing breathing, the skin and mucous membranes are often bright red, and other symptoms are aggravated; severe poisoning is manifested as loss of consciousness, tetanic and paroxysmal convulsions, opisthotonos, decreased blood pressure, urinary and fecal incontinence, often accompanied by cerebral edema and respiratory failure. Chronic effects are mainly manifested as neurasthenic syndrome, such as dizziness, headache, fatigue, a feeling of chest oppression, muscle pain, abdominal pain, etc., accompanied by irritation symptoms of the eyes and upper respiratory tract. After long – term skin contact, rashes may occur, manifested as macules and papules, and are extremely itchy.
The most commonly used and accurate method for detecting concentration and leakage is the “benzidine” method: prepare 0.1% benzidine acetate and 0.3% copper acetate solutions, store them separately in brown bottles, and mix 1 part of each solution before use (must be used within 15 minutes). Cut filter paper into small strips of 6cm×12cm and soak them in the solution. The paper strips with the solution will turn blue when exposed to hydrocyanic acid gas.
In terms of ventilation for dispersing poison and disposal of residues, after reaching the sealing time, open the air windows of the warehouse to disperse the poison. Personnel can enter only after the detection shows no toxicity. After the poison is completely dispersed, carry the reaction cylinder to a location more than 50m away from the warehouse, rivers, and wells and bury it deeply in a pit.
V. Poisoning Symptoms and First Aid Measures
(I) Poisoning Symptoms
| Poisoning Degree | Symptom Manifestations |
|---|---|
| Mild Poisoning | Irritation symptoms of the eyes and upper respiratory tract, headache, dizziness, chest tightness, nausea, weakness, etc., with a bitter almond smell in the exhaled breath. The symptoms can be relieved spontaneously. |
| Moderate Poisoning | Nausea, vomiting, a feeling of chest oppression, rapid breathing. The skin and mucous membranes are bright red or pale. |
| Severe Poisoning | Cause hydrocephalus in the brain, compress the nerves, lead to loss of consciousness, weakness, tetanic or paroxysmal convulsions, general muscle relaxation, disappearance of reflexes. The respiratory and heart functions may stop at any time. |
(II) First Aid Measures
On – site First Aid: Quickly transfer the patient to a place with fresh air, remove the contaminated clothes, and rinse the contaminated skin with water and 0.5% sodium thiosulfate. For those poisoned by ingestion, thoroughly wash the stomach with 0.2% potassium permanganate, 5% sodium thiosulfate, or 3% hydrogen peroxide. At the same time, keep the patient calm, warm, and provide oxygen inhalation.
Medical Treatment Measures: Adopt the sodium nitrite – sodium thiosulfate therapy. For mild and moderate patients, inhale amyl nitrite, inject 3% sodium nitrite intravenously in a timely manner, and then inject 10 – 20mL of 50% sodium thiosulfate. For severe patients, inject 2mL of 10% 4 – dimethylaminobenzoic acid intramuscularly, and then add 10g of sodium thiosulfate. If the symptoms recur, repeat half of the dose after 1 hour. During the treatment, provide oxygen inhalation. For patients with long – term coma and severe hypoxia, actively prevent and treat cerebral edema.







