Hydrogen Peroxide(7722-84-1)H2O2
Physical Properties of Hydrogen Peroxide
Hydrogen peroxide appears as a colorless liquid with a slight irritating odor and is transparent under normal temperature and pressure. Pure hydrogen peroxide is a light blue viscous liquid. Hydrogen peroxide has unique physical property parameters, as follows:
| Serial Number | Physical Property | Unit | Value |
|---|---|---|---|
| 01 | Melting Point | ℃ | -0.425 |
| 02 | Boiling Point | ℃ | 150.1 |
| 03 | Density | g/cm³ | 1.441 |
| 04 | Critical Temperature | ℃ | 457 |
| 05 | Critical Compression Factor | — | 0.358 |
| 06 | Dipole Moment | D | 2.26 |
| 07 | Standard Molar Combustion Enthalpy | kJ/mol | -53.7 |
| 08 | Molar Heat of Vaporization | kJ/mol | -46.674 |
| 09 | Standard Molar Gibbs Free Energy of Formation | kJ/mol | -120.35 |
| 10 | Standard Molar Enthalpy of Formation | kJ/mol | -187.78 |
| 11 | Standard Molar Entropy | kJ×kmol⁻¹×K⁻¹ | 109.6 |
| 12 | Standard API Gravity | ° | -33.6697 |
| 13 | Molar Mass | kg/kmol | 34.0147 |
Hydrogen peroxide follows the principle of “like dissolves like”. It can dissolve in polar substances such as alcohols and ethers, but is insoluble in non-polar substances such as benzene and petroleum ether. Additionally, hydrogen peroxide can be miscible with water in any proportion to form a hydrogen peroxide solution, commonly known as hydrogen peroxide solution. The common commercially available concentrations are 30% and 3% by mass. Moreover, the melting and boiling points of pure hydrogen peroxide molecules change with the change of its configuration. Due to its greater degree of association compared to water, it has a higher dielectric constant and boiling point.
Chemical Properties of Hydrogen Peroxide
The hydrogen peroxide molecule contains a peroxide bond (-O-O-), with a hydrogen atom connected to each end. The hydrogen atom and the oxygen atom are in different planes, and the angle between the two planes is 115°. The angle between the peroxide bond (red) and the hydrogen-oxygen bond (green) is 94.8°, and the bond lengths are 147.5 nm and 95 nm respectively.
Instability
Hydrogen peroxide is most stable in a weakly acidic environment (with a pH between 3.5 and 4.5). It is extremely prone to decomposition in an alkaline solution and also decomposes when exposed to strong light, especially short-wave radiation. Hydrogen peroxide forms explosive mixtures with many organic substances such as sugars, starches, alcohols, and petroleum products. It can explode when subjected to impact, heating, or electrical sparks. Although hydrogen peroxide itself is not flammable, it can react with combustible substances, releasing a large amount of heat and oxygen, which can cause ignition and explosion. H₂O₂ is extremely prone to decomposition into oxygen and water under conditions such as light irradiation, heating (decomposition is slow at room temperature, and it can decompose rapidly to cause an explosion above 153 ℃), the presence of certain metal salts (such as ferric chloride FeCl₃), metal oxides (manganese dioxide MnO₂), or rough active surfaces. Contact with many inorganic compounds or impurities can lead to rapid decomposition and explosion, releasing a large amount of heat, oxygen, and water vapor. Most heavy metals (such as iron, copper, silver, lead, mercury, zinc, cobalt, nickel, chromium, manganese, etc.) and their oxides and salts are active catalysts, and substances like dust, cigarette ash, carbon powder, and rust can also accelerate the decomposition. To inhibit the decomposition of hydrogen peroxide, on the one hand, the glass containers for storing hydrogen peroxide are mostly brown bottles. On the other hand, adding certain stabilizers such as trace amounts of sodium stannate (Na₂SnO₃) and sodium pyrophosphate (Na₄P₂O₇).
Strong Oxidizing Property
In an acidic environment (usually acidified with sulfuric acid H₂SO₄ and hydrochloric acid HCl), the oxidizing property of hydrogen peroxide is more prominent than in an alkaline environment, and it can act as a strong oxidant. The oxygen in the peroxide bond gains electrons and is reduced to more stable oxygen with a valence of -2 (H₂O). Here are some examples of hydrogen peroxide acting as a strong oxidant:
- H₂O₂ + 2I⁻ + 2H⁺ → I₂ + 2H₂O
- H₂O₂ + 2Fe²⁺ + 2H⁺ → 2Fe³⁺ + 2H₂O
- 4H₂O₂ + PbS → PbSO₄ + 4H₂O
Among them, lead sulfide is black, and lead sulfate is white. Therefore, H₂O₂ can be used for cleaning oil paintings, old furniture, and bleaching paper.
Weak Reducing Property
The oxidation state of oxygen in the peroxide bond is -1, which is between -2 (the lowest) and 0 (the highest). Therefore, hydrogen peroxide exhibits reducing properties (oxidized to O₂) when it comes into contact with stronger oxidants under specific circumstances. Take potassium permanganate (KMnO₄) and chlorine gas (Cl₂) as examples:
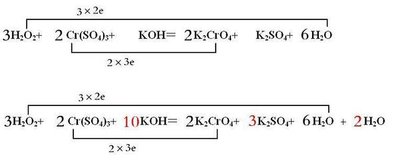
- 2KMnO₄ + 5H₂O₂ + 3H₂SO₄ → 2MnSO₄ + K₂SO₄ + 5O₂↑ + 8H₂O
- H₂O₂ + Cl₂ → 2HCl + O₂
Weak Acidity
Hydrogen peroxide is a dibasic weak acid and dissociates in two steps in an aqueous solution. Its first dissociation constant is relatively small (pKa1 = 11.64), and the second dissociation constant is even smaller (pKa2 = 25). It can undergo acid-base neutralization reactions with strong bases. Take sodium hydroxide (NaOH) as an example:
- H₂O₂ ⇌ H⁺ + HO₂⁻ Ka1 = 2.3×10⁻¹²
- HO₂⁻ ⇌ H⁺ + O₂⁻ Ka2 = 10⁻²⁵
- H₂O₂ + 2NaOH → Na₂O₂ + 2H₂O
Therefore, sodium peroxide (Na₂O₂) can be regarded as a salt of hydrogen peroxide to a certain extent.
Toxicological Data of Hydrogen Peroxide
The toxicological data of hydrogen peroxide are as follows:
- The LD50 is 2000 rag/kg (oral administration to mice).
- GRAS (Food and Drug Administration, §184.1366, 2000).
- The acceptable daily intake (ADI) is not specified (only used when there is no better way to preserve milk; Food and Agriculture Organization/World Health Organization, 2001).
Frequent exposure to hydrogen peroxide may lead to dermatitis, bronchial, and lung diseases. When poisoned by oral intake, symptoms such as abdominal pain, chest pain, dyspnea, vomiting, increased body temperature, bleeding of the conjunctiva and skin may occur. In some cases, visual impairment, convulsions, and paresis may also occur. In the United States, the usually specified maximum allowable concentration of hydrogen peroxide is 1.4 mg/m³.
China and chemical raw material suppliers, welcome to inquire,Contact us:https://www.yuhanchemi.com/contact