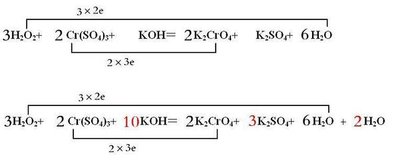
Hydrogen Peroxide/7722-84-1/H2O2
Diverse Application Scenarios
Medical Disinfection: A Gentle and Efficient Guardian of Health
In the medical field, a 3% hydrogen peroxide solution is a highly favored oxidizing disinfectant.
Compared to alcohol, it causes less irritation to the human body and boasts remarkable characteristics such as high efficiency, rapid action, and non – toxicity. When it comes into contact with organic substances, hydrogen peroxide decomposes to produce oxygen under the catalytic action of catalase, thereby achieving multiple functions including sterilization, deodorization, decontamination, and hemostasis.
Notably, it can effectively disrupt the anaerobic environment in which tetanus bacilli thrive, playing a significant role in preventing tetanus. Additionally, hydrogen peroxide solution is effectively used for anti – inflammatory treatment and cleaning in cases of otitis externa, tonsillitis, and emergency wound debridement.
Oxygen Generation: A Convenient Choice in Laboratories
Due to the decomposable nature of hydrogen peroxide, in a laboratory setting, oxygen can be conveniently produced by adding manganese dioxide (MnO₂) catalyst to a hydrogen peroxide solution.
This method is relatively easy to operate and has become one of the common ways to obtain oxygen in laboratories.
Forensic Science: A Scientific Aid in Cracking Clues
Hydrogen peroxide also has unique applications in forensic investigations. Catalase, present in biological cells, can catalyze the decomposition of hydrogen peroxide.
Forensic scientists utilize this property to roughly estimate the time of death of organisms. In addition, luminol, a luminescent reagent used for bloodstain detection, only emits light when treated with an oxidant.
A mixed aqueous solution of hydrogen peroxide and a hydroxide base is commonly used as the excitant, helping forensic investigators detect bloodstain clues that are otherwise imperceptible to the naked eye.
Dyeing and Bleaching: The Mainstay of Industrial Bleaching
In industrial production, hydrogen peroxide is a commonly used bleaching agent in the dyeing and textile industry.
It is widely applied to bleach various materials such as cotton fabrics, raw silk, wool, fur, feathers, hair, animal bones, ivory, fats, pulp, and tomb stones. For example, the pigments in some paints contain basic lead carbonate (molecular formula: 2PbCO₃·Pb(OH)₂).
After prolonged exposure to air, it reacts with hydrogen sulfide (H₂S) in the air, forming black lead sulfide (PbS) precipitates that adhere to the surface of furniture, making it look dull. However, scrubbing with a certain concentration of hydrogen peroxide solution can restore the original color of the furniture.
The principle is that sulfur in the – 2 valence state has strong reducibility and can undergo a redox reaction with H₂O₂, effectively removing the sulfide precipitates.
Organic Synthesis: A Green and Environmentally – Friendly Oxidizing Agent
Since the reduction product of hydrogen peroxide is only water, it is known as a green (strong) oxidizing agent and holds a significant position in the chemical industry related to oxidation reactions.
In the oxidative ring – opening reactions of aromatic hydrocarbons, the naphthalene ring undergoes ring – opening oxidation under the action of substances such as p – nitroaniline and p – nitrophenol, while the benzene ring is less likely to be oxidized, thus achieving the goal of selective oxidation. In the field of pesticide manufacturing, hydrogen peroxide is not only used in the production of pesticide 401 and thiram (tetramethylthiuram disulfide, with the chemical formula C₆H₁₂N₂S₄) fungicides but also plays a role in the oxidation of olefins to form epoxides.
The oxidation of ethylene to produce ethylene oxide has become one of the important industrial production methods (H₂C = CH₂ + H₂O₂ → CH₂OCH₂ + H₂O). In addition, organic peroxide peracetic acid (CH₃CO – OO – H) is industrially produced from acetic acid and hydrogen peroxide under the conditions of 45 – 55°C and 5 – 7kPa (CH₃COOH + H₂O₂ → CH₃COOOH + H₂O).
Inorganic Synthesis: An Important Raw Material for Enriching Inorganic Products
Hydrogen peroxide also has a wide range of applications in inorganic synthesis. It can be used to manufacture inorganic peroxides such as sodium perborate (NaBO₃) and sodium percarbonate (2Na₂CO₃·H₂O₂).
The chemical production methods of sodium perborate mainly include the boric acid method and the borax method (the borax method is the main one). The borax method uses borax (Na₂B₄O₇), sodium hydroxide, and hydrogen peroxide as raw materials, and sodium perborate is produced through three steps: the preparation of sodium metaborate (NaBO₂), the synthesis of sodium perborate, and the concentration of the mother liquor.
The production methods of sodium percarbonate are divided into the dry method and the wet method. The former uses anhydrous sodium carbonate and high – concentration hydrogen peroxide as raw materials to prepare sodium percarbonate crystals by stirring and mixing, while the latter involves adding hydrogen peroxide and sodium carbonate solutions sequentially in a reaction kettle and producing sodium percarbonate with the help of stabilizers.
In addition, hydrogen peroxide can be used to remove iron from inorganic salts and electroplating solutions. Through a series of chemical reactions (2Fe + 3H₂O₂ + 6H⁺ → 2Fe³⁺ + 6H₂O; 2Fe²⁺ + H₂O₂ + 2H⁺ → 2Fe³⁺ + 2H₂O; Fe³⁺ + 3OH⁻ → Fe(OH)₃↓), the purity of products and the quality of electroplated parts are improved.
Hydrogen peroxide with a mass fraction of over 90% can also be used as a raw material for fuels and explosives. During World War II, the German army used H₂O₂ as fuel to manufacture V – 1 and V – 2 rockets, and after the war, it has often been used as a high – energy oxidant and fuel for rocket fuels.
Storage and Transportation Standards
Storage Key Points
When storing hydrogen peroxide, it should be placed in a dedicated warehouse that is cool, dry, and well – ventilated, ensuring it is kept away from ignition sources and heat.
The warehouse temperature should not exceed 30°C, and the relative humidity should be controlled below 80%. Meanwhile, the containers should be kept sealed, and hydrogen peroxide should be stored separately from flammable (combustible) substances, reducing agents, active metal powders, etc., to avoid mixed storage.
In addition, the storage area should be equipped with emergency treatment equipment for leakage and suitable containment materials to handle potential leakage situations.
Transportation Requirements
During transportation, hydrogen peroxide should be shipped separately, ensuring that the containers do not leak, collapse, fall, or get damaged. Mixing it with acids, flammable substances, organic substances, reducing agents, spontaneous combustion substances, and substances that are flammable when wet is strictly prohibited. The transportation speed should not be too fast, and overtaking should not be forced. Transport vehicles should follow the specified routes. Before and after loading and unloading, the transportation vehicles should be thoroughly cleaned to prevent the mixing of impurities such as organic and flammable substances. At the same time, when workers come into contact with hydrogen peroxide during work, they should wear protective clothing, gloves made of polyethylene or polyvinyl chloride, and transparent protective goggles and masks made of polymer materials. In case of skin contact or splashing into the eyes, rinse immediately with warm water.
Regulatory Standards for Assurance
To ensure the safe and standardized use of hydrogen peroxide in various fields, a series of relevant regulatory standards have been formulated in China:
HG/T 5553 – 2019 Titanium Dioxide for Paper Industry, Hydrogen Peroxide for Soil Remediation, High – Purity Strontium Chloride and High – Purity Industrial Nitric Acid covers the relevant standards for hydrogen peroxide used in the paper industry, soil remediation, and other fields. These regulatory standards provide important bases for the production, use, and supervision of hydrogen peroxide, effectively ensuring its safe and efficient application in various fields
GB/T 1616 – 2014 (replacing GB 1616 – 2003) Industrial Hydrogen Peroxide clearly stipulates the quality and specifications of industrial hydrogen peroxide.
GB 22216 – 2008 Food Additive Hydrogen Peroxide standardizes the requirements for hydrogen peroxide used as a food additive.
GB/T 6684 – 2002 Chemical Reagent 30% Hydrogen Peroxide sets the standards for hydrogen peroxide used as a chemical reagent.
China and chemical raw material suppliers, welcome to inquire,Contact us:https://www.yuhanchemi.com/contact