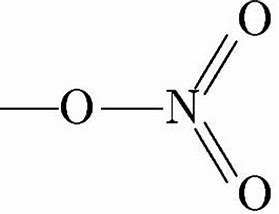
Nitric Acid/7697-37-2/HNO3
Detailed Explanation of Chemical Reactions and Safety Knowledge of Nitric Acid
Nitric acid, as an important chemical substance, is widely used in various fields. The following will introduce in detail its chemical reactions, toxicological prevention, and other aspects.
I. Chemical Reactions of Nitric Acid
(I) Esterification Reaction
Nitric acid can undergo an esterification reaction with alcohol to form the corresponding nitrate ester. In terms of the mechanism, in the past, it was believed that the esterification reaction involving nitric acid generated a carbocation intermediate. However, nowadays, many literatures describe its mechanism as the Fischer esterification reaction, that is, “the acid loses a hydroxyl group and the alcohol loses a hydrogen atom”, which is the same as the esterification mechanism of carboxylic acids. For example, in the preparation of nitroglycerin, the esterification reaction of nitric acid is used. In actual production, concentrated sulfuric acid is often used (it has a low cost and is easy to handle). Concentrated sulfuric acid can produce a large amount of NO₂. With other stronger dehydrating agents such as P₄O₁₀, a large amount of nitronium cations can also be produced, which is the essence of the nitration reaction. The esterification reaction of nitric acid is also used to produce nitrocellulose, and its reaction equation is: 3nHNO₃ + [C₆H₇O₂(OH)₃]n → [C₆H₇O₂(O – NO₂)₃]n + 3nH₂O.
(II) Nitration Reaction
Concentrated nitric acid or fuming nitric acid mixed with dehydrating agents (concentrated sulfuric acid, phosphorus pentoxide) can be used as a nitrating reagent to initiate the nitration reaction of some compounds. This reaction belongs to an electrophilic substitution reaction, and the electrophile is the nitronium ion. The dehydrating agent is beneficial for the generation of the nitronium ion. The most common nitration reaction is the nitration of benzene, and the reaction equation is: Ph – H + HO – NO₂ → Ph – NO₂ + H₂O.
(III) Oxidation-Reduction Reaction
The nitrogen element in the nitric acid molecule is in the highest valence state (+5), so nitric acid has strong oxidizing properties. Its reduction products vary depending on the concentration of nitric acid. Generally speaking, the higher the concentration of nitric acid, the fewer electrons each molecule of nitric acid gains on average. The reduction products of concentrated nitric acid are mainly nitrogen dioxide, and those of dilute nitric acid are mainly nitric oxide. More dilute nitric acid can be reduced to nitrous oxide, nitrogen, ammonium nitrate, etc. However, as the reaction proceeds and the concentration of nitric acid decreases, all possible reduction products may appear. The relevant potential diagram of nitric acid (under standard conditions, E/V) is as follows:
- HNO₃ – 0.798.9 → NO₂ – 1.08 → HNO₂ – 1.04 → NO – 1.582 → N₂O – 1.77 → N₂ – 0.27 → NH₄
- HNO₃ – 0.97 → NO
- HNO₃ – 1.25 → N₂O
- HNO₃ – 0.88 → N₂

(IV) Typical Reactions
- Reactions Involving Concentrated Nitric Acid
- Zn + 4HNO₃ ==== Zn(NO₃)₂ + 2NO₂↑ + 2H₂O
- P + 5HNO₃ ==== H₃PO₄ + 5NO₂↑ + H₂O
- Reactions Involving Dilute Nitric Acid
- 3Zn + 8HNO₃ ==== 3Zn(NO₃)₂ + 2NO↑ + 4H₂O
- 3P + 5HNO₃ + 2H₂O ==== 3H₃PO₄ + 5NO↑
- Reactions Involving Very Dilute Nitric Acid
4Zn + 10HNO₃ ==== 4Zn(NO₃)₂ + N₂O↑ + 5H₂O - Reactions Involving Extremely Dilute Nitric Acid
4Zn + 10HNO₃ ==== 4Zn(NO₃)₂ + NH₄NO₃ + 3H₂O
In addition, pure nitric acid can undergo autoionization: 2HNO₃ <==> H₂O + NO₂ + NO₃.
II. Toxicological Prevention of Nitric Acid
(I) Hazards
Nitric acid has multiple hazards. Contact with nitric acid vapor is very dangerous. Nitric acid liquid and vapor have a strong irritating and corrosive effect on the skin and mucous membranes. The nitrogen pentoxide released by the fumes of concentrated nitric acid forms an acid mist when it comes into contact with water vapor, which can be rapidly decomposed into nitrogen dioxide. The nitric acid vapor generated by heating concentrated nitric acid can also be decomposed into nitrogen dioxide, and inhalation can cause acute nitrogen oxide poisoning. When the concentration is lower than 12 ppm (30 mg/m³), there is no obvious damage to humans, and inhalation may cause pneumonia. The LC50 for rats inhaling nitric acid is 49 ppm/4 hours. Foreign cases show that for 3 people who inhaled nitric acid fumes, there were no respiratory symptoms in a short time. After 4 – 6 hours, progressive dyspnea occurred, and eventually, they died. Inhalation of nitric acid fumes can lead to acute poisoning, and oral intake of nitric acid can cause corrosive stomatitis and gastroenteritis, and may even lead to shock or renal failure.
Its hazard categories include acidic corrosives, oxidizing agents, explosive precursors, strong corrosives (when the content is higher than 70%) / oxidizing agents (when the content does not exceed 70%). The routes of entry are inhalation and ingestion. In terms of health hazards, inhaling nitric acid mist will irritate the respiratory tract and cause acute pulmonary edema; oral intake will cause severe abdominal pain, and in severe cases, gastric perforation, peritonitis, etc. may occur; contact with the eyes and skin will cause burns. Long-term contact may also cause dental erosion. At the same time, nitric acid is harmful to the environment, and it is a combustion-supporting substance. Mixing it with combustibles will cause an explosion.
(II) First Aid Measures
- Skin Contact: Immediately remove the contaminated clothing and rinse thoroughly with a large amount of running water for 20 – 30 minutes. Seek medical attention if there is any discomfort.
- Eye Contact: Immediately lift the eyelids and rinse thoroughly with a large amount of running water or normal saline for 10 – 15 minutes. Seek medical attention if there is any discomfort.
- Inhalation: Quickly move to an area with fresh air. Keep the respiratory tract unobstructed. If there is difficulty breathing, provide oxygen. If breathing and heartbeat stop, immediately perform cardiopulmonary resuscitation and seek medical attention as soon as possible.
- Ingestion: Rinse the mouth with water, drink milk or egg white, and then seek medical attention.
(III) Leakage Emergency Measures
For large amounts of leakage: Construct dikes or dig pits for containment. Absorb a large amount of liquid with fly ash or lime powder. Neutralize with agricultural lime (CaO), crushed limestone (CaCO₃), or sodium bicarbonate (NaHCO₃). Cover with antisolvent foam to reduce evaporation. Transfer to a tank truck or a special collector with a corrosion-resistant pump.
Designate a warning area according to the influence area of the liquid flow and vapor diffusion, and evacuate irrelevant personnel from the leeward and upwind directions to a safe area.
It is recommended that emergency treatment personnel wear positive-pressure self-contained breathing apparatus and acid-resistant clothing. All equipment used during the operation should be grounded. Do not touch the broken container and the leaked substance without wearing appropriate protective clothing.
Cut off the leakage source as much as possible. Prevent the leaked substance from entering water bodies, sewers, basements, or enclosed spaces.
Spray water mist to suppress the vapor or change the flow direction of the vapor cloud, and avoid contact between the water flow and the leaked substance. Do not allow water to enter the packaging container.
For small amounts of leakage: Cover the leaked substance with dry sand or other non-combustible materials.







