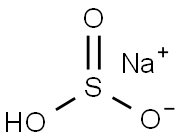
Sodium bisulfite 7631-90-5 NaHSO3
I. Chemical Properties of Sodium Bisulfite
Sodium bisulfite is usually a white monoclinic crystal with a sulfur dioxide odor. It is readily soluble in water and slightly soluble in alcohol. It is unstable in air and will slowly oxidize to sulfates. Its aqueous solution is weakly acidic. From a chemical structure perspective, it is an inorganic sodium salt with bisulfite as the counter ion. When it is heated and decomposed or comes into contact with inorganic acids, it will release highly toxic gaseous sulfur dioxide. It is a strong reducing agent and is incompatible with oxidants such as perchlorates and peroxides. It reacts with alkalis to form sulfates.
II. Diverse Applications of Sodium Bisulfite
(A) In the Industrial Field
Sodium bisulfite has a wide range of uses in industrial production. It can act as a reducing agent in industries such as dyes, papermaking, leather, and chemical synthesis. It is used for dyeing in the preparation of hot and cold indigo vats. In the papermaking process, it replaces sodium hyposulfite to remove Cl from bleached fibers. In washing, it serves as a stripping agent (reducing agent). It is also used in the manufacture of sodium dithionite, the coagulation of rubber latex, and as a preservative for deteriorating liquids or solutions for technical purposes. In the electroplating industry, it can be used as an additive for copper and brass electroplating. It also plays an important role in the digestion of wood pulp.
(B) In the Food Industry
Sodium bisulfite is a reducing bleaching agent permitted for use in China. It has a wide range of applications in the food field. It has a bleaching effect on food and can inhibit oxidase in plant-based foods. It can be used in various foods such as preserved fruits, glucose, and sugar. The maximum usage amount varies depending on the type of food. For example, the maximum usage amount in preserved fruits, dried fruits, etc. is 0.45g/kg, and in potato starch, it is 0.2g/kg. It can prevent food discoloration and inhibit bacterial growth. For instance, it is used in dried fruits to inhibit browning and maintain bright colors and is present in reconstituted lemon juice. In addition, it is used as a preservative and antioxidant in food and can also serve as a pharmaceutical adjuvant (antioxidant). However, it is not allowed to be used in meats and vitamin B source foods.
(C) Other Applications
In the analytical field, sodium bisulfite can be used as a reducing agent, bleaching agent, and bacterial inhibitor. In pharmaceutical products, it often serves as an antioxidant, disinfectant, or bleaching agent. It can also be used to remove permanganate stains from the skin and clothes. In the fermentation industry, it is used as a preservative. In winemaking, it is used as a disinfectant, bleaching agent, antioxidant, and an inhibitor of yeast and bacteria.

III. Production Processes of Sodium Bisulfite
There are mainly two production methods for sodium bisulfite. One method is to slowly add a sodium sulfite mother liquor containing 40% NaHSO₃ and with a pH value of 3 – 4 to soda ash to form a sodium sulfite solution (until the slurry has a pH value of 7 – 8). Then, transfer this solution to a series of reactors. Absorb the SO₂ gas (10% – 13%) produced by the combustion of sulfur to generate sodium bisulfite. During the reaction, a large amount of crystals will precipitate. After centrifugal separation, a wet substance with a water content of 6% – 10% is obtained. Finally, it is air-dried and dehydrated at 250 – 300°C to obtain the finished product. Another method is to use a soda ash solution to absorb the sulfur dioxide gas from the sulfuric acid production process to generate sodium bisulfite. Then, further centrifugal separation is carried out, and it is air-dried at 250 – 300°C to obtain the final product.
IV. Hazard and Safety Information of Sodium Bisulfite
(A) Hazardous Characteristics
Sodium bisulfite is a corrosive substance, and its toxicity is classified as moderately toxic. When heated, it can decompose into sulfur oxides. Its aqueous solution is acidic and corrosive. It has a strong irritant effect on the skin and tissues. The powder is irritating to the eyes, nose, and throat. Ingestion may irritate the stomach, and a large dose can cause severe colic, diarrhea, depression, and even death. It is a flammable/combustible material. It may catch fire when in contact with moist air or moisture. It may decompose explosively when heated or exposed to fire. It may reignite after being extinguished, and the container may explode when heated.
(B) Safe Storage and Transportation
In terms of storage and transportation, it should be stored in a warehouse with low temperature, good ventilation, and dry conditions. During transportation, the aqueous solution and the solid correspond to UN2693 and UN3260 respectively, and the hazard class is Class 8, with the label of 8 – Corrosive Materials.
(C) Emergency Treatment and Waste Disposal
When there is a fire, a large amount of water can be used as a fire extinguishing agent. In waste disposal, it can be poured into water, added with soda ash, and then neutralized with HCl, and finally flushed into the sewer with a large amount of water. At the same time, pay attention to its incompatibility with other substances during use to avoid dangerous reactions.
| Manufacturer | Product Description | CAS Number | Package | Price |
|---|---|---|---|---|
| yuhanchem | Sodium bisulfite for synthesis (39% aqueous solution) | 7631-90-5 | 1 liter | 38 US dollars |
| yuhanchem | Sodium bisulfite for synthesis (39% aqueous solution) | 7631-90-5 | 2.5 liters | 63.1 US dollars |
| yuhanchem | Sodium bisulfite for synthesis (39% aqueous solution) | 7631-90-5 | 25 liters | 399 US dollars |
| yuhanchem | Sodium bisulfite solution purum, ~40% | 7631-90-5 | 1 liter | 56.5 US dollars |
| yuhanchem | Sodium bisulfite solution purum, ~40% | 7631-90-5 | 2.5 liters | 116 US dollars |