Sulfuric Acid Formula: Understanding the H2SO4 Structure
Sulfuric acid (H₂SO₄), a colorless, oily liquid, stands as one of the most critical and extensively utilized chemicals worldwide. Its fundamental properties make it the backbone of countless industrial solutions, underpinning processes across a vast spectrum of sectors. For businesses relying on this essential compound, identifying dependable sulfuric acid manufacturers and suppliers is not merely a matter of procurement, but a cornerstone of operational efficiency, product quality, and safety.
- Sulfuric acid, denoted by the ubiquitous formula H2SO4, stands as a cornerstone chemical in a vast array of industrial processes, from fertilizer production to metal refining. While the concise H2SO4 formula provides a snapshot of its atomic composition, a deeper understanding of the intricate sulfuric acid structure is essential to truly grasp its diverse properties and potent reactivity. This comprehensive guide will dissect the chemical structure of H2SO4, meticulously exploring its atomic arrangement, the nature of its bonding, its characteristic molecular geometry, and the profound implications of its unique architecture.
Decoding the H2SO4 Formula: A Molecular Snapshot
The seemingly simple sulfuric acid formula, H2SO4, encapsulates the precise atomic makeup of a single molecule of this vital compound:
- Two (2) Hydrogen (H) atoms: These atoms are the source of sulfuric acid’s acidic behavior, capable of being donated as protons in chemical reactions.
- One (1) Sulfur (S) atom: Positioned as the central atom within the H2SO4 structure, sulfur belongs to Group 16 of the periodic table, known as the chalcogens.
- Four (4) Oxygen (O) atoms: These oxygen atoms are covalently bonded to the central sulfur atom, playing a crucial role in defining the molecule’s overall structure and its chemical reactivity.
The H2SO4 formula elegantly summarizes that these seven atoms are linked together through covalent bonds to form a distinct and functional molecule of sulfuric acid.
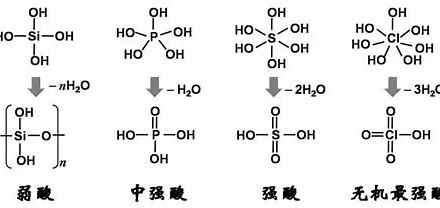
Unveiling the Sulfuric Acid Structure: A Tetrahedral Arrangement
The chemical structure of sulfuric acid is characterized by a central sulfur atom acting as a nexus, bonded to four surrounding oxygen atoms. Specifically, two of these oxygen atoms are connected to the sulfur via strong double bonds (S=O), while the remaining two oxygen atoms are linked through single bonds (S-O) and are each further bonded to a hydrogen atom, forming hydroxyl (-OH) groups. This specific arrangement of atoms around the central sulfur results in a characteristic tetrahedral molecular geometry.
- Central Sulfur Atom: The sulfur atom resides at the core of the H2SO4 structure, serving as the point of attachment for all four oxygen atoms. Its electronic configuration allows it to form more than the typical two bonds seen in oxygen, accommodating the four oxygen ligands.
- Sulfur-Oxygen Double Bonds (S=O): Two oxygen atoms engage in double bonds with the central sulfur atom. These double bonds are shorter and possess greater bond energy compared to single bonds, indicating a stronger attraction between the sulfur and oxygen atoms involved.
- Sulfur-Hydroxyl Groups (S-OH): The other two oxygen atoms are connected to the sulfur atom via single bonds. Each of these oxygen atoms is also bonded to a single hydrogen atom, forming the characteristic hydroxyl (-OH) functional groups. These hydroxyl groups are the key sites from which sulfuric acid can donate protons (H⁺), defining its acidic nature.
This tetrahedral structure around the sulfur atom is a common molecular geometry observed when a central atom is bonded to four other atoms and exhibits sp³ hybridization, where the sulfur atom’s atomic orbitals mix to form four equivalent hybrid orbitals that point towards the corners of a tetrahedron.
Exploring the Bonding in H2SO4
The bonds that hold the sulfuric acid structure together are predominantly covalent bonds, where adjacent atoms share pairs of electrons to achieve a more stable electron configuration.
- Covalent Bonds: The S=O double bonds involve the sharing of two pairs of electrons between the sulfur and oxygen atoms. Similarly, the S-O single bonds involve the sharing of one electron pair, and the O-H bonds within the hydroxyl groups are also formed through the sharing of an electron pair.
- Polar Covalent Bonds: Due to significant differences in electronegativity – the ability of an atom to attract shared electrons – between sulfur and oxygen, and between oxygen and hydrogen, these covalent bonds are polar. The more electronegative oxygen atoms attract the shared electrons more strongly, acquiring a partial negative charge (δ-), while the sulfur and hydrogen atoms bear partial positive charges (δ+). This inherent polarity within the H2SO4 structure is crucial for its solubility in polar solvents like water and its ability to participate in hydrogen bonding with water molecules.
- Absence of Sulfur-Hydrogen Bonds: It is a crucial structural feature of H2SO4 that hydrogen atoms are not directly bonded to the sulfur atom. Instead, they are always attached to an oxygen atom within the hydroxyl (-OH) groups. This arrangement is fundamental to its acidic behavior.
Visualizing the H2SO4 Lewis Structure
The Lewis structure provides a valuable visual representation of the valence electrons and the bonding arrangement within the sulfuric acid molecule. In its most common and accurate representation:
- The sulfur (S) atom is positioned at the center.
- Two oxygen (O) atoms are connected to the sulfur via double bonds (S=O), each oxygen possessing two lone pairs of electrons.
- Two hydroxyl (-OH) groups are connected to the sulfur via single bonds (S-O). The oxygen atom in each hydroxyl group carries two lone pairs of electrons and is further single-bonded to a hydrogen (H) atom. Each hydrogen atom shares its single electron with the oxygen atom.
While resonance structures can be drawn to represent the electron delocalization in sulfuric acid, the depiction with two distinct S=O double bonds and two S-OH single bonds accurately reflects the connectivity and charge distribution within the molecule.
The Significance of the H2SO4 Structure: Diprotic Acid
The specific structure of sulfuric acid directly underpins its classification as a diprotic acid. The presence of the two hydroxyl (-OH) groups is key, as each of these groups contains a hydrogen atom that can be donated as a proton (H⁺) when H2SO4 is dissolved in aqueous solutions. This proton donation occurs in two distinct and sequential steps, reflecting the two acidic protons in the molecule:
- First Dissociation (Strong): H₂SO₄(aq) → H⁺(aq) + HSO₄⁻(aq)
- Second Dissociation (Weaker): HSO₄⁻(aq) ⇌ H⁺(aq) + SO₄²⁻(aq)
The ability to donate two protons, a defining characteristic of a diprotic acid, is a direct consequence of the two hydroxyl groups being integral components of the H2SO4 structure.
Key Takeaways: Understanding the H2SO4 Architecture
- The sulfuric acid formula, H2SO4, signifies the presence of two hydrogen atoms, one sulfur atom, and four oxygen atoms within each molecule.
- The sulfuric acid structure exhibits a central sulfur atom with a tetrahedral geometry, bonded to two oxygen atoms via double bonds and to two hydroxyl (-OH) groups via single bonds.
- The chemical bonding within H2SO4 is primarily polar covalent, contributing to its properties and reactivity.
- The Lewis structure provides a visual representation of the atomic arrangement and the distribution of valence electrons within the molecule.
- The presence of two hydroxyl (-OH) groups in the H2SO4 structure is the fundamental reason why it behaves as a diprotic acid, capable of donating two protons in solution.
In conclusion, a comprehensive understanding of the sulfuric acid formula and its corresponding three-dimensional structure is not merely an academic exercise. It is foundational to comprehending the chemical behavior and the remarkably wide-ranging applications of this vital compound across numerous industrial and laboratory settings. The specific arrangement of atoms, the nature of their bonds, and the resulting molecular geometry directly dictate sulfuric acid’s strong acidic properties and its ability to participate in a multitude of essential chemical reactions.
yuhan Sodium Chlorate:https://www.yuhanchemi.com/sodium-chlorate
We support product customization Specific specifications, grades, reagents, price comparison is welcome
Contact us:https://www.yuhanchemi.com/contact