Urea Hydrogen Peroxide (UHP) /124 – 43 – 6/ CH₆N₂O₃
Basic Information
The urea hydrogen peroxide complex is a complex formed by the strong hydrogen bond between urea and hydrogen peroxide. It is in the form of white crystals, with a melting point of 90-93°C, and is easily soluble in water, ethanol, ethylene glycol, dichloromethane and other organic solvents.
Chinese Name: Urea Hydrogen Peroxide Complex
English Name: Urea hydrogen peroxide adduct
Alias: Carbamide perhydrate, Carbamide-Peroxide
Chemical Formula: CH₆N₂O₃
Molecular Weight: 94.07
CAS Number: 124 – 43 – 6
Computational Chemical Data
| Classification | Details |
|---|---|
| Reference value of hydrophobic parameter calculation (XlogP) | None |
| Number of hydrogen bond donors | 4 |
| Number of hydrogen bond acceptors | 3 |
| Number of rotatable chemical bonds | 0 |
| Number of tautomers | 2 |
| Topological molecular polar surface area | 110 |
| Number of heavy atoms | 6 |
| Surface charge | 0 |
| Complexity | 29 |
| Number of isotope atoms | 0 |
| Number of determined atomic stereocenters | 0 |
| Number of undetermined atomic stereocenters | 0 |
| Number of determined chemical bond stereocenters | 0 |
| Number of undetermined chemical bond stereocenters | 0 |
| Number of covalent bond units | 2 |
Properties and Stability
- Basic Properties: The aqueous solution has the properties of both urea and hydrogen peroxide, and can slowly release oxygen in water. Compared with inorganic peroxides such as sodium percarbonate, its performance is more superior, with the advantages of high active oxygen content, high solubility in water, good stability, and solubility in organic solvents.
- Storage Conditions: The wet reagent will decompose into water and oxygen above 40°C, which is corrosive and oxidizing. It is recommended to store it in a dry and cool place.
Storage Method
Store in a dry, clean, well-ventilated warehouse at a room temperature of 15°C to 25°C. Keep away from ignition sources, heat sources and avoid direct sunlight. Pay attention to moisture prevention and rain protection.
Synthesis Method
- Wet Process and Dry Process: The dry process requires a relatively high concentration of hydrogen peroxide (about 85(wt)%), and has the disadvantages of complex equipment, harsh technical conditions, high energy consumption, and poor product stability. Most of the preparation of carbamide peroxide adopts the wet process, that is, using a low concentration of hydrogen peroxide to react with a saturated or supersaturated urea solution, adding a certain amount of stabilizer and controlling the reaction temperature, and obtaining the product through filtration and drying, and recycling the mother liquor.
- Recrystallization Method: Prepare by recrystallizing urea in a 30% aqueous solution of hydrogen peroxide.
Applications
- Pharmaceutical and Pharmaceutical Industry: Urea peroxide can be used as an efficient, safe and convenient solid disinfectant, and can also be used as an aqueous solution. Compared with hydrogen peroxide and peracetic acid, it has obvious advantages such as strong bactericidal power, wide bactericidal spectrum, low use concentration, and no residual toxicity. It can also inhibit the growth of bacteria and molds, and the residue is non-irritating. It is used for treating liver ascites in cancer treatment.
- Daily Chemical Industry: Urea peroxide can be used as a bleaching agent, straightening agent, perming and hair dye neutralizer for human and animal hair. Especially when added to toothpaste, it can play a role that ordinary toothpaste cannot achieve: reducing dental plaque and bacteria, reducing periodontal diseases and dental caries. At the same time, it can harden the tooth enamel and have a strong inhibitory effect on dental caries. In addition, urea peroxide can be used as a bleaching agent in neutral detergents.
- Agriculture and Aquaculture Industry: Urea peroxide can be used as an oxygenator, disinfectant for fish ponds and an emergency agent when there is a lack of oxygen in the pond in the aquaculture industry. It can also be used as a disinfection and oxygen supply agent for small poultry feed. It can also be used as an oxygen-enriched ripening agent for fruits and vegetables.
- Textile and Paper Industry: Urea peroxide can be used as a bleaching agent for cotton, wool, rayon and linen fibers. It can be used as a softener, antistatic agent and decolorizing agent for polyamide fibers. It is also used as a bleaching agent in the paper industry and an ore flotation improver.
- Organic Synthesis: Urea hydrogen peroxide complex (UHP) is a complex formed by the strong hydrogen bond between urea and hydrogen peroxide. It transforms unstable hydrogen peroxide into a solid that is easy to operate, safe and stable at room temperature, and is inexpensive. UHP shows different oxidation abilities under different solvents and catalysts, and is an important oxidation reagent in organic synthesis.
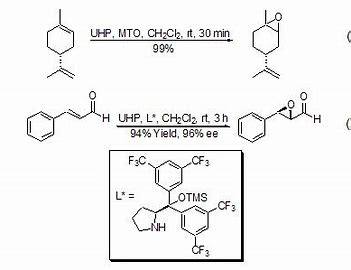
- Epoxidation of Double Bonds: UHP has the properties of hydrogen peroxide and is often used in the epoxidation of double bonds, and has high regional and stereoselectivity and high conversion rate. UHP can be used in combination with acid anhydrides (such as acetic anhydride and maleic anhydride) to generate organic peroxyacids in situ, and this method can replace the use of high-concentration organic peroxyacids. For the epoxidation of α,β-unsaturated ketones and benzoquinones, the catalysis of a base, such as K₂CO₃, is generally required. UHP in combination with methyltrioxorhenium (MTO) makes the epoxidation of olefins have excellent regional and stereoselectivity.
- Asymmetric Oxidation of α,β-Unsaturated Aldehydes: For the asymmetric oxidation of α,β-unsaturated aldehydes, UHP shows better stereoselectivity and conversion rate than other oxidants.
- Conversion of Functional Groups: Since the oxidation performance of UHP can be easily regulated, it can be used for the conversion of various functional groups, for example: converting cyano groups into amides, oximes into nitro groups, and aldehydes into acids, etc. Another important use of UHP is the oxidation reaction of heteroatoms such as N, S, P, I and Si. In most cases, its selectivity is better than that of aqueous hydrogen peroxide.
- Baeyer-Villiger Rearrangement Reaction: UHP, like organic peracids, can also complete the Baeyer-Villiger rearrangement reaction, and has high stereoselectivity.
- Oxidation of Inactive C-H Bonds and Iodination of Aryl Groups: Recent literature reports that UHP can also achieve the oxidation of inactive C-H bonds to form hydroxyl or ester groups; UHP in combination with I₂ can conveniently and effectively achieve the iodination of aryl groups.







