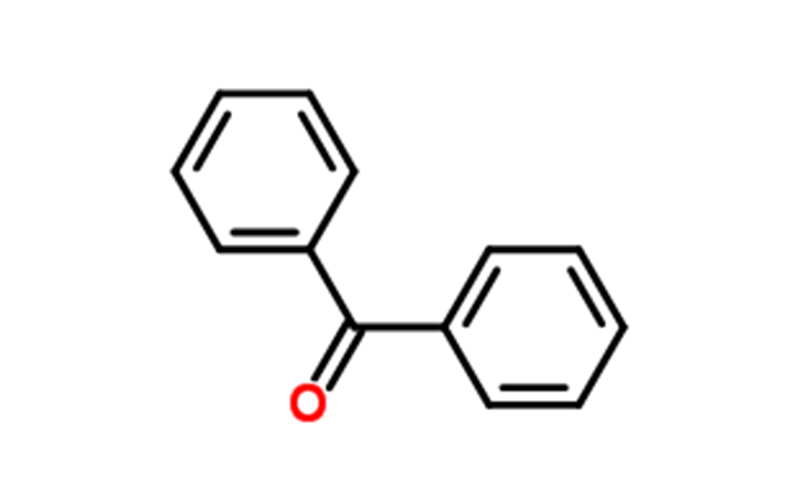
Benzophenone Chemical Properties
| Melting point | 47-51 °C (lit.) |
| Boiling point | 305 °C (lit.) |
| density | 1.11 |
| vapor density | 4.21 (vs air) |
| vapor pressure | 1 mm Hg ( 108 °C) |
| refractive index | 1.5893 |
| Fp | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | ethanol: soluble100mg/mL, clear, colorless (80% ethanol) |
| form | Crystalline Powder or Flakes |
| color | White to off-white |
| Odor | Characteristic. |
| Odor Type | balsamic |
| Water Solubility | insoluble (<0.1 g/100 mL at 25 ºC) |
| Merck | 14,1098 |
| JECFA Number | 831 |
| BRN | 1238185 |
| Dielectric constant | 13.0(20℃) |
| Stability: | Stable. Incompatible with strong oxidizing agents, strong reducing agents. Combustible. |
| InChIKey | RWCCWEUUXYIKHB-UHFFFAOYSA-N |
| LogP | 3.18 at 25℃ |
Key Characteristics:
- Photoinitiator: Effectively initiates free-radical polymerization reactions when exposed to ultraviolet (UV) light.
- UV Absorber (UV Filter): Absorbs harmful UV radiation, protecting products from photodegradation.
- Solubility (Benzophenone Solubility):
- Slightly soluble in water (generally considered almost insoluble).
- Highly soluble in various organic solvents, such as alcohol (ethanol), acetone, ether, acetic acid, chloroform, and benzene.
- Stability: Stable under appropriate storage conditions.
Primary Uses and Functions of Benzophenone
- What is benzophenone used for? What is benzophenone’s function? Benzophenone uses and properties.
- A. High-Efficiency Photoinitiator in UV Curing Applications:
- As a typical Type II photoinitiator, benzophenone efficiently generates free radicals under UV light (especially UV-C wavelengths), initiating the polymerization of monomers or oligomers for rapid curing.
- Specific Application Examples:
- Printing Inks and Coatings: Widely used in UV-curable inks (e.g., for packaging, labels) and UV-curable varnishes/coatings (e.g., for wood, flooring, automotive refinish paints). Benzophenone ensures these inks and coatings cure rapidly under UV lamps, forming hard, durable surfaces and improving production efficiency and product quality.
- Adhesives: A key component in photoreactive adhesives, such as those used in dental fillings, electronic component encapsulation, and certain industrial bonding applications, enabling fast curing and strong adhesion.
- 3D Printing: In some UV-cured resin 3D printing processes, benzophenone may be part of the photoinitiator system, facilitating the rapid layer-by-layer curing of materials.
- B. Excellent Ultraviolet (UV) Absorber/Stabilizer:
- By absorbing UV radiation and converting it into harmless heat, benzophenone and its derivatives effectively protect various materials and products from UV-induced degradation.
- Specific Application Examples:
- Plastics and Polymers: Added to transparent plastic products like PET plastic bottles (e.g., for mineral water, beverages), polyolefin (PE, PP) films, and polycarbonate (PC). It prevents the plastic itself from yellowing or becoming brittle due to UV exposure and protects the contents (e.g., juices, cooking oils, milk) from photo-oxidation, extending shelf life. For instance, without benzophenone as a UV blocker, many transparent beverage bottles would have to be opaque or dark-colored.
- Cosmetics and Personal Care Products: Functions as a UV filter to protect fragrances, colors, and active ingredients in perfumes, lotions, shampoos, lipsticks, and sunscreens (e.g., benzophenone-3, or oxybenzone, as an active sunscreen ingredient) from UV damage, stabilizing formulations and helping to protect skin from UV radiation.
- Paints, Varnishes, and Coatings: Improves the UV resistance of outdoor-use architectural coatings, automotive paints, and furniture varnishes, preventing fading, chalking, and cracking, thereby extending their lifespan and aesthetic appeal.
- C. Fragrance Ingredients and Food Additives:
- Fragrances and Flavors: Used as a fixative and enhancer in fragrances, lending a sweet, woody, geranium-like note. For example, in soaps, detergents, and lower-cost perfumes, it helps stabilize and boost the scent, prolonging its longevity.
- Food Additives: Functions as a flavor enhancer in specific food products. For instance, it is permitted by the FDA in limited quantities in certain non-alcoholic beverages and candies to impart a specific flavor profile.
- D. Pharmaceutical Intermediate and Research:
- What is benzophenone used for in pharmaceuticals?
- Key Intermediate and Raw Material: In the pharmaceutical industry, benzophenone is widely used as a crucial intermediate for synthesizing various complex chemicals and drug compounds. For example, it forms part of the starting material or structural backbone for certain anti-inflammatory drugs, antimicrobials, or anticancer medications.
- Analytical Standard: In drug development and quality control, benzophenone can serve as a reference standard for analytes in pharmaceutical formulations, used in techniques like High-Performance Liquid Chromatography (HPLC) and spectrophotometry for qualitative and quantitative analysis, ensuring drug purity and content.
- Biophysical Probe: In biological research applications, benzophenone’s photoactivatable properties make it useful as a photophysical probe for identifying and mapping peptide-protein interactions, aiding in understanding protein structure and function.
Toxicity Classification and Carcinogenicity
Benzophenone is not a phenol. Phenols are compounds with a hydroxyl group (–OH) directly attached to the aromatic ring, whereas benzophenone is a diaryl ketone, and its structure does not contain a hydroxyl group directly connected to the aromatic ring.
IARC Classification: The International Agency for Research on Cancer (IARC) has classified benzophenone as a Group 2B carcinogen, which means it is “possibly carcinogenic to humans.” This classification is based on evidence of liver, kidney, and blood cell tumors observed in experimental animals such as rats and mice, but the evidence of carcinogenicity in humans is limited and further research is ongoing. For example, Proposition 65 in California also lists benzophenone as a chemical known to cause cancer.
Organ Toxicity: Long-term or repeated exposure, especially through oral exposure, may cause damage to the liver and kidneys. For example, the European Food Safety Authority (EFSA) has classified benzophenone as a known toxic substance because it can cause liver enlargement in rats at lower doses.
Common Exposure Routes and Health Impacts
Skin Contact: Although it is commonly found in cosmetics, prolonged or repeated contact with pure benzophenone may cause skin irritation and dryness. Some individuals may experience phototoxicity or contact dermatitis as allergic reactions.
Eye Contact: Direct contact may cause severe eye irritation.
Inhalation: Inhalation of high concentrations of benzophenone dust or vapor may cause respiratory tract irritation, accompanied by dizziness, mild headache, nausea, and loss of coordination. For example, during the application of UV-cured floor coatings or certain coatings, if ventilation is poor, there may be a risk of inhalation exposure.
Ingestion: Accidental ingestion of large amounts of benzophenone may cause damage to the liver and kidneys. For example, there have been past reports of benzophenone migrating from food packaging inks into food (although this is now strictly regulated).
Endocrine Disruption: Some studies suggest that benzophenone and its metabolites (such as 4-hydroxybenzophenone) may have weak estrogenic or antiandrogenic activity, indicating potential endocrine-disrupting properties.
Our company specializes in hazardous chemicals, flammable and explosive chemicals, toxic chemicals (legal export), ultra-pure and high-purity reagents. Welcome to contact us.
Packing and shipping