What Color Does Lithium Chloride Burn? (Crimson Flame Explained)
How Electrons Emit Light in a Flame Test
At the core of every flame test lies the dynamic movement of electrons. These tiny particles orbit an atom’s nucleus in specific energy levels, much like rungs on a ladder. When you introduce a sample, say lithium chloride, to a flame, its electrons absorb energy from the heat. This surge of energy causes them to “jump” to a higher, more energetic rung.
But this excited state is temporary. Just like a spring recoiling, these electrons quickly return to their lower energy state, known as the “ground state.” As they fall back, they release the absorbed energy in the form of light. The specific color of this light is directly determined by the amount of energy released during this transition.
For example, lithium’s electrons release energy that corresponds to a vibrant crimson red hue, which is why lithium chloride produces such a rich red flame. This process is universal for all elements, yet the colors vary due to their unique electron configurations and the specific energy differences involved.
Why Flame Colors Are Uniquely Different for Each Element
Not all flames look the same, and this is because each element possesses a distinctive “fingerprint” of light. This unique signature is defined by the specific energy levels available to its electrons. No two elements have identical electron arrangements, meaning the precise amount of energy released—and consequently, the resulting color—is unique to each atom.
Here’s how this works:
- Diverse Electron Transitions: Consider sodium, whose electrons release energy that results in a vivid bright yellow flame. In spectroscopy, this intense yellow light from sodium primarily results from its outer electrons transitioning from the 3p energy level back to the 3s level, emitting specific wavelengths. In contrast, lithium’s electrons produce that characteristic crimson, which is typically due to its electrons transitioning from the 2p to the 2s energy level.
- The Impact of Energy Difference: The greater the energy gap between the excited state and the ground state, the higher the energy of the light emitted. Higher-energy transitions manifest as colors like blue or violet, while lower-energy transitions typically yield reds and yellows.
This fundamental principle is why flame tests are incredibly effective for identifying metal ions. The colors act as unique visual identifiers, like name tags for the elements, providing a simple yet powerful analytical tool. Every flicker in a flame test offers a direct reflection of atomic structure, providing a tiny window into the very nature of matter.
How are flame tests so effective at identifying metal ions?
Flame tests are effective at identifying metal ions because each type of metal atom has a unique electron configuration and energy level structure. When these atoms are excited in a high-temperature flame, their electrons transition in specific ways and emit light of particular wavelengths. These wavelengths combine to form the element’s unique “spectral fingerprint,” which is what we perceive as the flame’s color.
Here are some examples illustrating their effectiveness:
- Introducing sodium chloride (NaCl) to a flame immediately produces a very strong and persistent bright yellow flame. This yellow is so dominant it can even mask weaker colors from other elements present.
- When we introduce potassium chloride (KCl) to a flame, we see a pale lilac (or violet) flame. This color is usually much weaker than sodium’s yellow and sometimes requires viewing through a cobalt blue glass to filter out potential interference from trace amounts of sodium.
- Calcium chloride (CaCl2) yields a distinctive brick-red or orange-red flame.
- Strontium chloride (SrCl2) displays a vivid bright red or scarlet flame.
- Barium chloride (BaCl2) emits a characteristic apple-green flame.
- Copper chloride (CuCl2) typically produces a blue-green color.
It’s these distinct and reproducible colors that make flame tests a quick, intuitive, and cost-effective preliminary qualitative analytical method in chemistry, especially for identifying alkali metal and alkaline earth metal ions.
The Science Behind Lithium Chloride’s Vivid Crimson Flame
When lithium chloride is introduced to a flame, it produces a stunning crimson red color – a phenomenon deeply rooted in atomic science. This vivid display perfectly illustrates how thermal energy interacts with an atom’s electrons, resulting in a unique and recognizable hue.
Lithium’s Signature Crimson: A Distinctive Shade
Upon exposure to a flame, lithium chloride burns with its distinctive crimson or magenta-red flame. This striking hue is a direct result of lithium atoms absorbing heat energy, which excites their electrons to higher energy states. As these electrons then return to their lower energy levels, they emit light specifically within the red spectrum.
Contrasting this with other common elements’ flame colors further highlights lithium’s uniqueness:
- Sodium emits an intense and prevalent bright yellow, often called “sodium yellow.” This is because sodium atom electron transitions primarily release energy in the yellow wavelength range, making even trace amounts highly noticeable.
- Potassium displays a pale lilac (violet), which is much softer than sodium’s yellow and often harder to observe, as it can be masked by the strong yellow light from sodium.
- Calcium’s flame is brick-red or orange-red, slightly more orange than lithium’s crimson.
- Strontium also emits red, but it’s typically a vibrant bright red, sometimes more scarlet than lithium’s crimson.
It is this specific, almost magenta-tinged crimson red that makes lithium’s flame color stand out among elements, serving as its unique “signature light.”
The Crucial Role of Neutral Lithium Atoms
You might wonder why it’s not lithium ions creating the light. In fact, it’s the neutral lithium atoms that are responsible for this vibrant light emission. When the energy from the flame excites these neutral lithium atoms, their electrons jump to higher energy states. It is upon their return to their original, lower energy states that these electrons release photons (light particles). This emitted light is what produces the distinctive red glow we observe. Neutral atoms, not ions, are essential for generating light in this process because ions lack the specific electron energy transitions that lead to visible light emission in flame tests.
This crucial interaction is further explained in this science overview of lithium flame tests.
Understanding Lithium Electron Energy Levels
Electrons are not static within an atom; they jump between specific, quantized energy levels. Imagine these levels as distinct steps on a staircase—each corresponding to a precise amount of energy. When lithium chloride encounters the heat of a flame, its electrons readily absorb this energy and leap to these higher energy levels. However, this elevated state is unstable. Consequently, they quickly “fall” back down to their original positions, releasing light as they settle.
Here’s the captivating part: the precise distance between these energy levels within the lithium atom dictates the color of the emitted light. For lithium, this specific energy gap corresponds to a wavelength within the red spectrum, which perfectly explains its characteristic and vibrant crimson flame. Exploring the physics of lithium’s energy transitions can provide a deeper understanding.
Understanding these atomic movements reveals the intricate beauty and scientific precision behind flame colors. Every flicker in the flame is a direct result of the atomic structure of lithium, making it as scientifically significant as it is visually captivating.

Practical Applications of Flame Test Colors
Flame test colors do more than just dazzle the eyes—they offer invaluable practical uses in both educational and recreational settings. Whether in a classroom or at a grand fireworks display, these unique hues serve as powerful tools, informing and entertaining in equal measure.
Educational Demonstrations
Flame tests are a cornerstone of chemistry education, captivating students with their vibrant colors while providing hands-on lessons in atomic theory. Schools and laboratories frequently conduct flame tests to illustrate the fundamental concepts of energy levels and electron transitions.
In these controlled experiments, students directly observe how specific metal salts—such as sodium chloride (producing bright yellow) or lithium chloride (producing crimson)—yield distinct flame colors. By associating these unique colors with specific elements, learners grasp critical concepts about the intricate relationship between energy, electrons, and light. The engaging, hands-on nature of these tests makes complex atomic principles more relatable, effectively sparking enthusiasm for science.
For educators seeking a more structured approach, incorporating flame test kits or adhering to established guidelines, like those outlined in this flame test demonstration resource, can streamline the activity while ensuring both safety and engagement. Beyond teaching basic atom-electron interactions, these tests also emphasize the analytical aspect of chemistry, preparing students for more advanced studies in spectroscopy by showcasing the transition from simplified flame colors to sophisticated diagnostic tools used in laboratories worldwide.
Fireworks and Visual Effects
If you’ve ever been mesmerized by a fireworks show, you’ve witnessed flame test colors put to stunning, large-scale use. Metal salts, including lithium chloride, play a starring role in creating the brilliant hues we associate with pyrotechnic displays. For example, lithium chloride’s crimson glow is a key contributor to the deep reds seen in many fireworks, painting the night sky with dramatic tones.
Here’s how it works: Firework shells contain small packets of different metal salts. When ignited, the intense heat causes these salts to emit their characteristic colors. Each hue—from copper’s vibrant blue to sodium’s intense yellow and barium’s striking green—is a direct result of flame test principles applied on a massive scale. This guide on fireworks chemistry delves deeper into how these colors are meticulously engineered.
Lithium’s vivid red flame is particularly valued in pyrotechnics, often used to create powerful and dramatic visual effects. When combined with other elements, fireworks designers can layer and blend colors for truly breathtaking displays. The science extends beyond mere beauty: pyrotechnicians meticulously select compounds to ensure both safety and reliability in their spectacular creations.
Flame test colors also find their brilliance in other visual effects, such as colored campfire kits and stage productions. These applications rely on the same fundamental principles, transforming basic chemical reactions into captivating visual experiences.
This crucial interaction is explained further in this science overview of lithium flame tests.
Flame test colors also shine in other visual effects, such as colored campfire kits and stage productions. These applications rely on the same principles, transforming basic chemistry into mesmerizing art for audiences worldwide. For a fun activity on how flame test colors relate to fireworks, try this STEM project guide.
China and chemical raw material suppliers, welcome to inquire,Contact us:https://www.yuhanchemi.com/contact

Compound Fertilizer
Compound Fertilizer
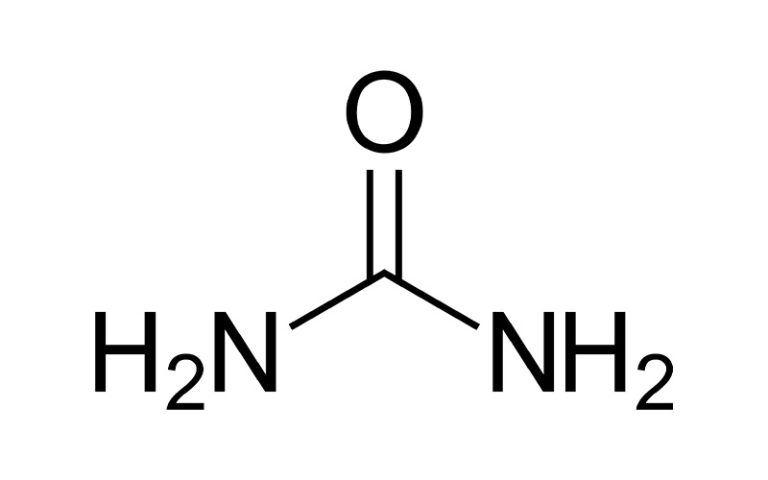
Urea
CAS No.: 57-13-6
Molecular formula: CH₄N₂O
Sodium Citrate: A “Miracle” Ingredient with Versatility and Safety
What is Sodium Citrate? In modern life, many products we encounter daily hide some “behind-the-scenes heroes,” and sodium citrate is one of them. Item Information English Name Sodium Citrate CAS Number 68-04-2 (anhydrous); 6132-04-3 (dihydrate) Molecular Formula C₆H₅Na₃O₇ (anhydrous); C₆H₅Na₃O₇·2H₂O (dihydrate) Molecular Weight 258.07 (anhydrous); 294.10 (dihydrate) Appearance Colorless crystals or white crystalline powder Odor…

Citric Acid: Healthier Use in Food
It’s ubiquitous, yet it often sparks curiosity about its safety and origin. Is citric acid “good” or “bad”? What are its amazing uses? How does it differ from lemon juice? And is it true that some link it to black mold? Item Details Chemical Formula C₆H₈O₇ Molar Mass Approximately 192.12 g/mol IUPAC Name 2-Hydroxypropane-1,2,3-tricarboxylic acid…
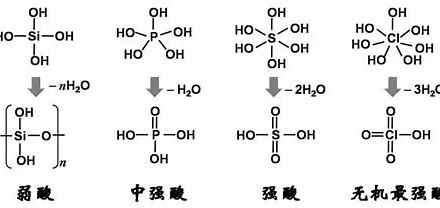
Phosphoric Acid (PA)H₃PO₄
1. Phosphoric Acid Overview 1.1 What is Phosphoric Acid? Phosphoric acid, with the chemical formula H3PO4, is an inorganic acid and an important oxyacid of phosphorus. It commonly exists as a viscous liquid, is colorless, odorless, and possesses moderate acidity. Phosphoric acid is stable at room temperature, non-volatile, and its CAS number is 7664-38-2. 1.2…

Тетрапотассиевая пирофосфат (TKPP) промышленного класса
1. Обзор продукта 1.1 Краткое описание Тетрапотассовый пирофосфат (TKPP) представляет собой белый порошок или частицы, которые легко растворимы в воде и являются эффективным хелатообразователем, отличным диспергатором, стабильным эмульгатором и точным регулятором рН.Производительность TKPP промышленного класса, которую мы обеспечиваем, стабильна и надежна, что делает ее идеальным выбором для удовлетворения ваших требовательных потребностей в промышленном применении. 1.2…






