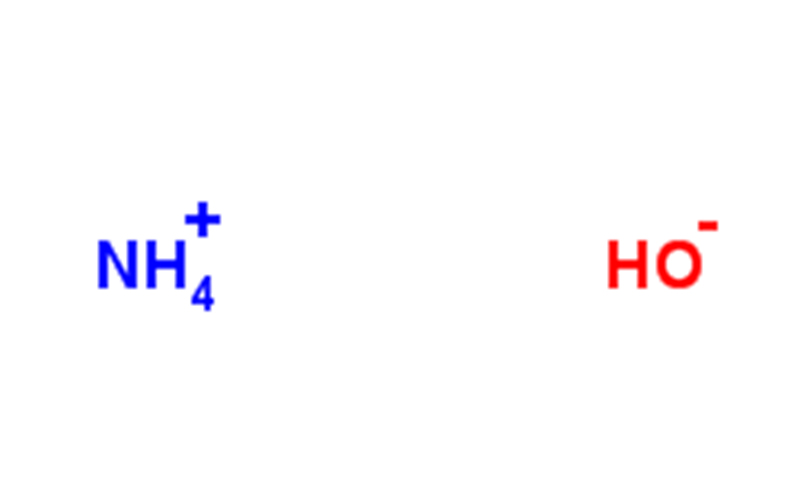Product Details:
| parameter | United States | minimum | utmost | |
| Assay (NH3) | % | 28.0 | 30.0 | |
| color | APA | 5 | ||
| Chloride (Cl) | ppm | 0.01 | ||
| Carbonate (CO3) | ppm | 1 | ||
| Sulfate (SO4) | ppm | 0.01 | ||
| Phosphate (PO4) | ppm | 0.01 | ||
| Reducing substance, permanganate (in KMnO4) | ppm | 0.5 | ||
| Impurity maximum | United States | minimum | utmost | |
| Aluminum (Al) | ppb | 0.05 | ||
| Antimony (Sb) | ppb | 0.05 | ||
| Arsenic (As) | ppb | 0.05 | ||
| Barium (Ba) | ppb | 0.05 | ||
| Beryllium (Be) | ppb | 0.05 | ||
| Bismuth (Bismuth) | ppb | 0.05 | ||
| Boron (B) | ppb | 0.05 | ||
| Cadmium (Cd) | ppb | 0.05 | ||
| Calcium (Ca) | ppb | 0.05 | ||
| Chromium (Cr) | ppb | 0.05 | ||
| Cobalt (Co) | ppb | 0.05 | ||
| Copper (Cu) | ppb | 0.05 | ||
| Gallium (Ga) | ppb | 0.05 | ||
| Germanium (Ge) | ppb | 0.05 | ||
| Gold (AU) | ppb | 0.05 | ||
| Iron (iron) | ppb | 0.05 | ||
| Lead (Pb) | ppb | 0.05 | ||
| Lithium (Li) | ppb | 0.05 | ||
| Magnesium (Mg) | ppb | 0.05 | ||
| Manganese (Mn) | ppb | 0.05 | ||
| Molybdenum (Mo) | ppb | 0.05 | ||
| Nickel (Ni) | ppb | 0.05 | ||
| Niobium (Nb) | ppb | 0.05 | ||
| Potassium (K) | ppb | 0.05 | ||
| Silver (Ag) | ppb | 0.05 | ||
| Sodium (Na) | ppb | 0.05 | ||
| Strontium (Sr) | ppb | 0.05 | ||
| Tantalum (Ta) | ppb | 0.05 | ||
| Thallium (Tl) | ppb | 0.05 | ||
| Tin (Sn) | ppb | 0.05 | ||
| Titanium (Ti) | ppb | 0.05 | ||
| Vanadium (V) | ppb | 0.05 | ||
| Zinc (Zn) | ppb | 0.05 | ||
| Zirconium (Zr) | ppb | 0.05 | ||
| Particle count | United States | minimum | utmost | |
| ≧ 0.1 microns | pcs/ml | 200 | ||
| ≧ 0.2 microns | pcs/ml | 50 | ||
| ≧ 0.5 microns | pcs/ml | 30 | ||
| ≧ 1.0 microns | pcs/ml | 10 | ||
Physical and chemical properties
- Density: ~0.88 g/cm³ at 20°C
- Boiling Point: ~36°C (as a function of concentration)
- Melting Point: ~-77°C
- pH: Strongly alkaline (pH >11)
- Solubility: Completely miscible with water, reacts with acids
- Decomposition: Ammonia gas (NH₃) is released over time, especially when heated
The difference between ammonia water and ammonia monohydrate
1. Ammonia monohydrate
Ammonia monohydrate is a pure substance, chemical formula: NH3· H2O is a weak base. Ammonia is mostly dissolved in water to form ammonia monohydrate, NH3+H2O⇌NH3· H2O。 It is the main component of ammonia (ammonia is a mixture), and it is easy to volatilize and escape ammonia. It has a strong pungent odor and is miscible with ethanol. Corrosive, tear-jerking. Ammonia monohydrate is a weak electrolyte, which can be partially ionized into ammonium ions and hydroxide ions (so it is sometimes called ammonium hydroxide) in water, and is weakly alkaline: NH₃· H₂O====(NH₄⁺)+(OH⁻)
2. Ammonia
Ammonia water, also known as ammonia water, is mainly composed of NH3· H2O, an aqueous solution of ammonia, is colorless, transparent and has a pungent odor. Ammonia water is a mixture, which is a mixture of ammonia gas soluble in water, i.e., an aqueous solution of ammonia monohydrate. Industrial ammonia is an aqueous solution containing 25%~28% ammonia, and only a small part of ammonia molecules in ammonia react with water to form ammonia monohydrate, which is a weak base that only exists in ammonia.
Ammonia contains the ions NH₄⁺, NH₂⁻, OH⁻ and H₃O⁺. Ammonia dissolved in water gives ammonia monohydrate with Kb = 1.8×10-5 for this reaction. The pH of 1M ammonia is 11.63, and about 0.42% of NH₃ becomes NH₄+.
Nature difference
1. Properties of ammonia monohydrate
Ammonia monohydrate has a certain corrosive effect. The corrosion of copper is relatively strong, the steel is relatively poor, and the corrosion to cement is not great. It also has a certain corrosive effect on wood. Ammonia monohydrate is unstable and easy to decompose to form ammonia and water. Because ammonia is volatile, ammonia should be kept sealed in a brown or dark reagent bottle with a rubber stopper and placed in a cool, dark place.
2. Properties of ammonia
Ammonia is alkaline and is a good precipitating agent, which can react with a variety of metal ions to form insoluble weak bases or amphoteric hydroxides. Ammonia also exhibits weak reducing properties and can be oxidized by strong oxidants. Only a small part of the ammonia molecules react with water to form ammonium ions NH4+ and hydroxide ions OH-, which are weakly alkaline. Ammonia can react with acids to form ammonium salts. White smoke is produced when ammonia gas volatilizes from concentrated ammonia meets acid mist volatilized by volatile acids such as concentrated hydrochloric acid and concentrated nitric acid.
Key Applications
- Semiconductor industry:
- Used in RCA cleaning (SC-1) to remove organic matter and particle contamination from wafers
- Essential in etching and chemical-mechanical planarization (CMP) processes
- Electronics Manufacturing:
- High-purity alkaline etchants for microelectronics and display panels
- Key reagents in photoresist stripping
- Chemistry and Drug Synthesis:
- For high-purity chemical formulations
- Precursors to ammonium-based compounds in research and industry
- Environmental and analytical applications:
- Used for ultrapure water production and wastewater treatment
- Reagents in ICP-MS and other trace analysis techniques
Safety and handling
Ammonia, Ammonia, NH3, colorless gas. Has a strong pungent odor. Density 0.7710. Relative density 0.5971 (air=1.00). It is easy to liquefy into a colorless liquid. It can be liquefied by pressurizing at room temperature (critical temperature 132.4°C, critical pressure 11.2 megapascals, i.e. 112.2 atmospheres). Boiling point -33.5°C. It is also easy to solidify into a snow-like solid.
Melting point -77.75°C. Soluble in water, ethanol and ether. At high temperatures, it will decompose into nitrogen and hydrogen, which has a reducing effect. In the presence of catalysts, it can be oxidized to nitric oxide. It is used to make liquid nitrogen, ammonia, nitric acid, ammonium salts and amines. It can be made by the direct synthesis of nitrogen and hydrogen, which can burn the mucous membranes of the skin, eyes, and respiratory organs, and if people inhale too much, it can cause lung swelling and even death.
Chemical properties
Corrosive
Ammonia has a certain corrosive effect, and ammonia carbonization is more corrosive. The corrosion of copper is relatively strong, the steel is relatively poor, and the corrosion to cement is not great. It also has a certain corrosive effect on wood. It is a hazardous chemical, and the hazard regulation number is 82503.
Weakly alkaline
The following chemical equilibriums exist in ammonia:
NH3+H2O===NH3· H2O (Reversible Reaction)
NH3· H2O===NH4+ +OHˉ (reversible reaction) ionization constant: K=1.8×10ˇ-5 (25°C)
Therefore, only a small part of the ammonia molecule reacts with water to form the ammonium ion NH4+ and the hydroxide ion OH-, so it is weakly alkaline.
(1) It can make the colorless phenolphthalein test solution red, the purple litmus test solution blue, and the wet red litmus test paper blue. This method is commonly used in laboratories to test for the presence of NH3.
(2) It can react with acids to form ammonium salts. White smoke is produced when concentrated ammonia meets volatile acids such as concentrated hydrochloric acid or concentrated nitric acid. However, there is no such phenomenon in the case of non-volatile acids (such as sulfuric acid, phosphoric acid). Therefore, this method can be used to test the presence of ammonia molecules in water in the laboratory. In industry, the weak alkalinity of ammonia is used to absorb sulfuric acid industrial exhaust gas and prevent environmental pollution.
instability
Ammonia monohydrate is unstable, sees light, and is easily decomposed by heat to form ammonia and water.
In the laboratory, ammonia can be produced by heating a solid mixture of calcium hydroxide and ammonium chloride or mixing concentrated ammonia water with solid caustic soda at room temperature, which is easy to set up and operate, and the ammonia concentration obtained is larger, and the effect of the “fountain” experiment is better.
Because ammonia is volatile and unstable, ammonia should be kept tightly sealed in a brown or dark reagent bottle in a cool, dark place.
Precipitation
Ammonia is a good precipitant that reacts with a variety of metal ions to form insoluble weak bases or amphoteric hydroxides.
complexity
When a small amount of ammonia is produced, an insoluble weak alkali is produced, and when the ammonia is excessive, the insoluble substances are converted into complex ions and dissolved.
Reducibility
Ammonia exhibits weak reducing properties and can be oxidized by strong oxidants.
Oxidizing
Hydrogen in the +1 oxidation state of the ammonia molecule exhibits weak oxidation and can oxidize strong reducing agents.