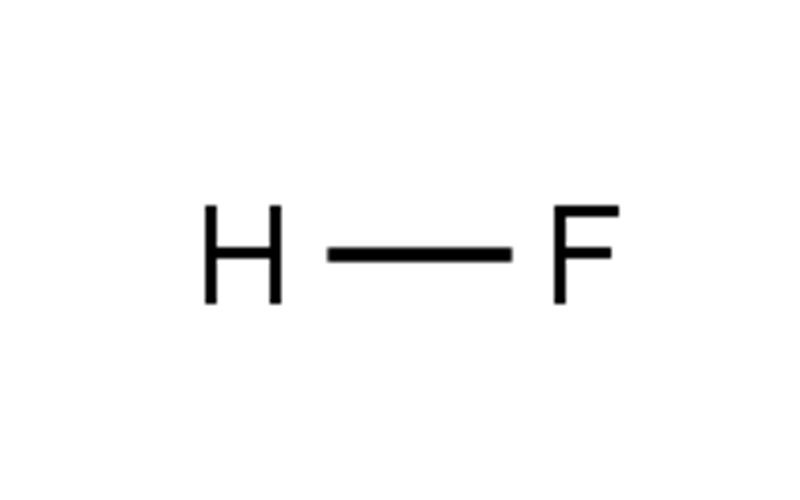Product details/Korean ultra-pure hydrofluoric acid parameters
| parameter | at | minimum | maximum | |
| Detection (HF) | % | 48.8 | 49.2 | |
| color | APHA’s | 10 | ||
| Nitrate (NO3) | ppb | 15 | ||
| Chloride (Cl) | ppb | 15 | ||
| Phosphate (PO4) | ppb | 15 | ||
| Sulfates and sulfites (SO3 and SO4) | ppb | 15 | ||
| The impurities are the greatest | at | minimum | maximum | |
| Aluminum (Al) | ppb | 0.05 | ||
| Antimony (Sb) | ppb | 0.05 | ||
| Arsenic (As) | ppb | 0.05 | ||
| Barium (Ba) | ppb | 0.05 | ||
| 铍 (Be) | ppb | 0.05 | ||
| Bismuth (Bi) | ppb | 0.05 | ||
| Boron (B) | ppb | 0.05 | ||
| Cadmium (Cd) | ppb | 0.05 | ||
| Calcium (Ca) | ppb | 0.05 | ||
| Chromium (Cr) | ppb | 0.05 | ||
| Cobalt (Co) | ppb | 0.05 | ||
| Copper (Cu) | ppb | 0.05 | ||
| 镓 (Ga) | ppb | 0.05 | ||
| Germanium (Ge) | ppb | 0.05 | ||
| Gold (AU) | ppb | 0.05 | ||
| Iron (Fe) | ppb | 0.05 | ||
| 铟 (In) | ppb | 0.05 | ||
| Lead (Pb) | ppb | 0.05 | ||
| 锂 (Li) | ppb | 0.05 | ||
| Magnesium (Mg) | ppb | 0.05 | ||
| Manganese (Mn) | ppb | 0.05 | ||
| Molybdenum (Mo) | ppb | 0.05 | ||
| Nickel (Ni) | ppb | 0.05 | ||
| 铌 (Nb) | ppb | 0.05 | ||
| Potassium (K) | ppb | 0.05 | ||
| Silver (Ag) | ppb | 0.05 | ||
| Sodium (Na) | ppb | 0.05 | ||
| Strontium (Sr) | ppb | 0.05 | ||
| 钽 (Ta) | ppb | 0.05 | ||
| 铊 (Tl) | ppb | 0.05 | ||
| Tin (Sn) | ppb | 0.05 | ||
| Titanium (Ti) | ppb | 0.05 | ||
| 钒 (V) | ppb | 0.05 | ||
| Zinc (Zn) | ppb | 0.05 | ||
| Zirconium (Zr) | ppb | 0.05 | ||
| Particle count | at | minimum | maximum | |
| ≧ 0.1 microns | pcs/ml | 300 | ||
| ≧ 0.2 microns | pcs/ml | 150 | ||
| ≧ 0.5 microns | pcs/ml | 25 | ||
Hydrofluoric acid is generally divided into two categories: industrial grade hydrofluoric acid and electronic grade hydrofluoric acid according to purity and use, of which electronic grade hydrofluoric acid is further subdivided into multiple grades according to purity, the following is a specific introduction:
Industrial grade hydrofluoric acid
- Purity: Hydrogen fluoride content ≥40%, iron content ≤0.01%, sulfuric acid content ≤0.02%, arsenic content ≤2%.
- Usage: Mainly used in chemical, glass, ceramics and other industries, such as glass etching, ceramic processing, metal surface treatment, etc.
Electronic grade hydrofluoric acid
Electronic grade hydrofluoric acid is divided into the following five levels according to purity, and the regular data is adjusted according to concentration:
| level | SEMI standards | Metal impurity content | Control particle size | Applicable IC line width range | Applicable IC integration | Main applications: |
|---|---|---|---|---|---|---|
| THE | C1/C2 | ≤100ppb | ≤1μm | >1.2μm | 1M、4M | Small and medium-scale integrated circuit and electronic component processing |
| UP | C7 | ≤10ppb | ≤0.5μm | 1.0μm | 1G、4G、16G | 1μm integrated circuit and TFT-LCD manufacturing |
| UPS | C8 | ≤1ppb | ≤0.2μm | 0.35~0.8μm | 256M | Micron integrated semiconductor integrated circuits |
| UPSS | G5 | ppt level | ≤0.2μm | 0.09~0.2μm | Advanced process | High-end semiconductor manufacturing |
| UPSSS | G5 | ppt level | ≤0.2μm | <0.09μm | Advanced process | High-end semiconductor manufacturing |
Level description
- EL grade: The content of metal impurities is less than 100ppb, controlling 1 micron particle size particles, reaching the SEMI C1/C2 standard, suitable for small and medium-scale integrated circuits and electronic component processing processes.
- UP grade: suitable for 1 micron integrated circuit and TFT-LCD manufacturing process, metal impurity content less than 10ppb, filtered by 0.2 micron pore filter, controlled 0.5 micron particles, filled in a 100-level purification environment, to meet SEMI C7 standard.
- UPS grade: suitable for 0.35-0.8 micron integrated circuit processing process, metal impurity content less than 1ppb, filtered by 0.05 micron pore size filter, controlled 0.2 micron particles, filled in a level 100 purification environment, to meet SEMI C8 standard.
- UPSS grade: semiconductor grade, suitable for the production of 0.09~0.2μm and <0.09μm IC process technology, is the most advanced level, only a few enterprises such as polyfluorine can produce and large-scale application in China.
- UPSSS grade: It is the highest purity grade available, and the metal impurity content reaches the ppt level, which is suitable for the most advanced semiconductor manufacturing processes.
Production method
1. Sulfuric acid method: the dried fluorite powder and sulfuric acid are mixed according to the ratio of 1:(1.2~1.3
), and sent to the rotary reactor for reaction, and the gas phase temperature in the furnace is controlled at 280 °C±10 °C. After the reaction, the gas enters the crude distillation column, and most of the sulfuric acid, water and fluorite powder are removed, and the temperature of the tower kettle is controlled at 100~110 °C, and the temperature at the top of the column is 35~40 °C. The crude hydrogen fluoride gas is then condensed into a liquid state by the degassing tower, the temperature of the tower kettle is controlled at 20~23 °C, the top temperature of the tower is -8 °C±1 °C, and then it enters the distillation tower for rectification, the temperature of the tower kettle is controlled at 30~40 °C, and the temperature at the top of the tower is 19.6 °C±0.5 °C. After refining, hydrogen fluoride is absorbed with water, that is, hydrofluoric acid products are obtained.
2. The industrial-grade hydrofluoric acid is purified by distillation, condensed and separated to remove impurities, and the dust particles are removed by microporous filter membrane filtration to obtain colorless and transparent electronic-grade hydrofluoric acid.
3. Using hydrogen fluoride in the cylinder industry as raw material, use a polyethylene pipe to connect the washing bottle, open the cylinder valve, and let the hydrogen fluoride gas slowly pass into the absorber equipped with qualified conductive water to analyze pure hydrofluoric acid.
4. Using industrial hydrofluoric acid as raw material, first use potassium permanganate to oxidize the organic matter and sulfurous acid in the raw material, and then add potassium carbonate (or potassium fluoride and barium carbonate) according to the impurity content in the raw material to remove impurities such as sulfuric acid, fluorosulfonic acid, fluorosilicic acid and hydrochloric acid (the generated non-volatile matter is left at the bottom layer). Stir evenly, stand still, stir again, after the reaction is complete, the temperature is distilled, and the distillate is collected in a clean polyvinyl chloride container, which is the reagent hydrofluoric acid.
5. The production of hydrofluoric acid has two raw material routes: fluorite and phosphate rock. The sulfuric acid method with fluorite as raw material is a common method used in industry. Hydrofluoric acid is obtained by mixing dried fluorite powder and sulfuric acid in a ratio of 1:(1.2~1.3
) and by high temperature decomposition, crude distillation, rectification and water absorption.
quality
- Density: ~1.45 g/cm³ at 20°C
- Boiling point: Decomposition before boiling (~150°C at 1 atmosphere)
- Melting point: -0.43°C
- pH: slightly acidic (pH of 4.5–5.5 for diluted solution)
- Solubility: miscible with various proportions of water, slightly soluble in alcohols and ethers
- Decomposition: Slowly decomposes into water and oxygen under the acceleration of heat, light, and impurities
Main applications:
- Semiconductor industry:
- For wafer cleaning processes (RCA cleaning)
- It is essential for removing organic and metal contaminants from microelectronics
- Pharmaceuticals and Biotechnology:
- Sterilization of equipment and facilities
- It is used in the synthesis of pharmaceutical intermediates
- Precision Electronics Manufacturing:
- Etching process for printed circuit boards (PCBs).
- Oxidizing agents in high-precision chemical reactions
- Chemical synthesis:
- Key reagents in oxidation reactions
- Production of high-purity chemical intermediates
- Environmental Applications:
- Wastewater treatment to remove contaminants
- Advanced Oxidation Process (AOP) for Air and Water Purification
Both hydrofluoric acid and molten sodium hydroxide can be used to remove the glass coating layer on the surface of microfilaments, and the removal time of the glass coating with a thickness of 10 μm is about 150 s at room temperature, and it takes about 10 s to melt sodium hydroxide.
The composition and structure of glass are important factors affecting the corrosion resistance of glass-coated pure copper microwires.
The removal of the glass coating layer on the surface of the microfilament was experimentally studied, the corrosion behavior of the microfilament in hydrofluoric acid and molten sodium hydroxide was evaluated, the corrosion resistance of the glass-coated pure copper microwire in strong acid and alkali was analyzed, and the corrosion mechanism was discussed.
Security and handling
- Hazards: Strong oxidizing agent, which can cause severe burns and eye damage. It can react violently with organic materials, metals and reducing agents.
- Storage:
- Store in a cool, dry, well-ventilated place
- Store in a non-reactive container (usually made of HDPE or Teflon)
- Avoid exposure to sunlight and heat
- Protective gear: Use goggles, gloves, and a lab coat. In industrial settings, ensure proper ventilation or use a fume hood.